Clinical Infectious Diseases

News
04.02.2026: Congratulations! Prof. Christoph Lange awarded the 2025 Oskar Medical Prize
26.01.2026: DanGerouS Mycobacteria Club met in Malmö in 2026
15.01.2026: Congratulations on your habilitation, Priv. Doz. Dr. Thomas Theo Brehm!
12.01.2026: Non-invasive diagnosis of tuberculosis from respiratory masks
22.12.2025: Increased Risk of Tuberculosis After Organ Transplantation in Europe
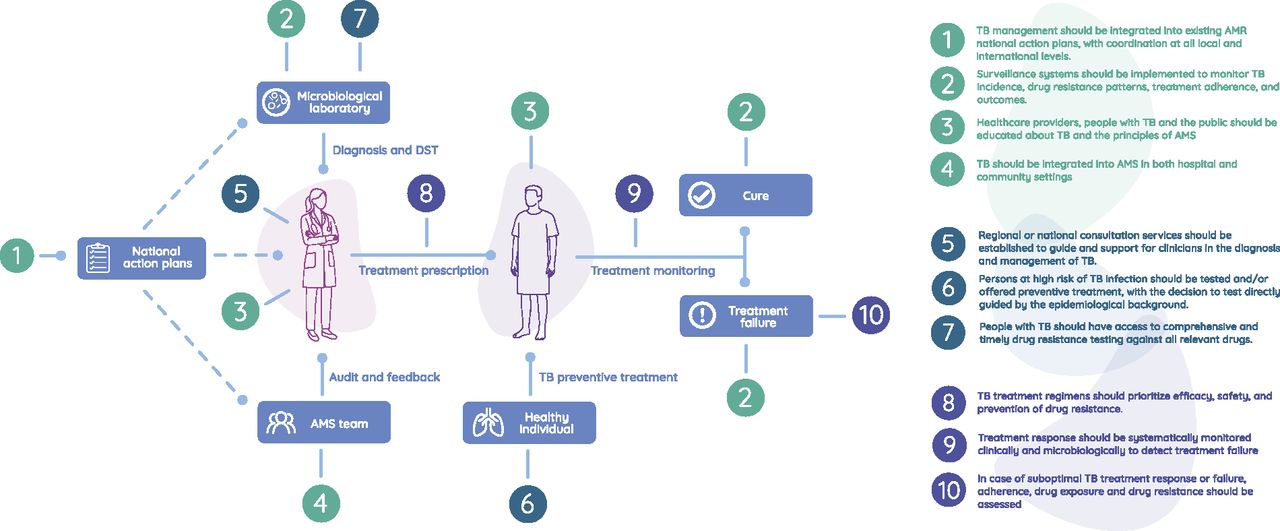
19.12.2025: New Clinical Standards Strengthen Antimicrobial Stewardship in Tuberculosis Care
We aim to substantially improve the prevention, diagnosis and treatment of tuberculosis and other respiratory infectious diseases and to integrate scientific advances into clinical practice.
Highlights
UNITE4-TB: Clinical lead, coordinator of European trial sites and leader in biomarker development in EU-industry funded Phase II international trials platform for the development of new TB regimens
TB-X: Identification and validation of biomarkers to predict responses to TB therapy, to predict the occurrence of adverse events and to individualize the duration of TB treatment.
Drug-resistant TB: Epidemiological surveys and evaluation of molecular tools to identify Mycobacterium tuberculosis drug-resistance in adults and children, development of novel antibiotics and international management guidelines for the treatment of drug-resistant tuberculosis.
DZIF (German Center for Infection Research)
- DZIF Clinical Tuberculosis Centre (Clin TB)
- German TB Cohort / TB-samples Bio-Repository
- Eastern European Study Site (EESS)
- Pediatric Diagnostic Cohort
- Therapeutic Drug Monitoring (TDM)
- Risk Score
- DZIF MD projects
- Implementation and evaluation of the targeted Next-Generation Sequencing (tNGS) assay ‘FreezeTB’ for drug-resistant tuberculosis in the Republic of Moldova
BMG (Federal Ministery of Health) - GHPP (Global Health Protection Programme)
- Targeted Next-Generation Sequencing (tNGS) for drug-resistant tuberculosis (TB) in the Republic of Moldova
BMZ (Federal Ministery for Economic Cooperation and Development) and ekfs (Else Kröner-Fresenius-Stiftung)
- BREATHE
EU-Projects
- UNITE4TB
TBnet-Projects
- TBnet e. V. - General information
- Evaluation of an escape room as an interprofessional teaching and learning tool
- Consensus documents on TB management among migrants in Europe
- TBnet-database
Lower Saxony Association for the Fight against Tuberculosis, Lung and Bronchial Diseases e.V.
- Biomarker-based diagnosis of tuberculosis (detecTB)
In-house Studies
- Evaluation of a diary for assessing treatment outcomes in patients with pulmonary infections caused by nontuberculous mycobacteria
- Immunregulatory function of the PD-1/ PD-L1-checkpoint pathway in tuberculosis
- Post-Tuberculosis-Lung-Disease (PTLD) in Albania (ASTRA)
- Post-tuberculosis- and biomarker-study in Moldova (TBpredict)
- CRISPR-based measurement of cell-free DNA
- Complication rate of outpatient parenteral antimicrobial therapy of infection with non-tuberculous mycobacteria (NTM)
- Prediction of adverse events in tuberculosis treatment
- DRAMATIC
- Potential of lipoarabinomannan (LAM) for the detection of tuberculosis
S-H Society for the Prevention & Control of Tuberculosis & Lung Diseases e.V.
- Access TMDmetrics
- Validation of a new biomarker (TB22) for the diagnosis and therapy monitoring of tuberculosis
- Epigenetic and genetic analysis of treatment outcomes in patients with multidrug-resistant and drug-susceptible tuberculosis
- Impact of sputum-based and sequence-guided individualized therapy on treatment outcomes in drug-resistant TB (T3 study)
Borstel-Baylor Cooperation
- Analysis of M. tuberculosis-DNA from stool for the diagnosis of tuberculosis
- CHIPS-TB22
- Impact of tNGS in eSwatini
- Siyakhula
- INSIGHT
- TB Gaps
The clinical infrastructure (ClinTB) for tuberculosis research at the German Center for Infection Research (DZIF) has been operating since 2013 for the development and implementation of new scientific methods to improve patient care, further education and training and the development of clinical guidelines.
The University of Lübeck and the research center have been supporting the DZIF since 2014 with the establishment of the W3 professorship for Respiratory Medicine & International Health.
Our laboratory comprises approx. 100 m2 of modern infrastructure for molecular biological and immunological analysis. There is close cooperation with the national reference laboratory for mycobacteria and other scientific laboratories on our campus.
The spectrum of clinical research ranges from prevention to diagnostics and therapy. In basic scientific work, we investigate mechanisms of resistance and susceptibility to infections with Mycobacterium tuberculosis and we develop methods to improve the diagnosis of tuberculosis and to predict the response to therapy. Together with colleagues from other clinics, universities and research institutes, we are conducting national and international cohort studies to quantify the risk of developing tuberculosis in different populations and to evaluate diagnostic procedures to predict the development of active tuberculosis.
We are involved in international projects to develop new tuberculosis drugs and to identify and evaluate biomarkers that will enable therapy monitoring and, if necessary, individualized therapy duration.
In close collaboration with colleagues from other research groups at the RCB, we are developing methods for personalized medicine for tuberculosis. We translate information about mutations in the genome of Mycobacterium tuberculosis, results of differentiated antibiotic resistance determinations of the pathogens and analyses of antibiotic concentrations in the blood of patients into customized therapies. We coordinate europe-wide observed cohort studies with the aim of improving the management of multidrug-resistant tuberculosis (MDR-TB).
In addition to the clinical aspects, we are interested in the socio-medical circumstances that lead to differences in the quality of care for tuberculosis patients and health-associated migration due to tuberculosis diseases within Europe.


In tuberculosis research, we rely on cooperation with international research networks, such as the Tuberculosis Network European Trialsgroup (TBnet), the Collaborative Group for the Meta-Analysis of Individual Patient Data in MDR-TB treatment and in EU-funded projects, currently ClickTB (EDCTP), Stool4TB (EDCTP) and the Unite4TB consortium.
With a group of experienced doctors, our team conducts around 700 documented telephone consultations (TBinfo: +49 (0)4537 188 0) on all aspects of tuberculosis and infections with non-tuberculous mycobacteria every year.
Regular training and further education events include the “Clinical Tuberculosis Course”, which takes place in Borstel at the end of each spring (registration via +49 (0)4537 188 7080). The course combines state-of-the-art lectures on the management of tuberculosis with an update on important new developments and includes international focus topics. Every three years, the course is held in English for an international audience.
We are regularly involved in the preparation of national and international guidelines and reviews on tuberculosis, diseases caused by non-tuberculous mycobacteria and pulmonary mycoses and are represented on advisory committees of the World Health Organization (WHO) and Médecins Sans Frontières (MSF).
There is close cooperation with the University Medical Center Hamburg Eppendorf in teaching and clinical research.
To improve TB control globally, reduce TB-associated morbidity, and favorably influence individual disease courses, a more personalized approach to TB care is needed—an approach that considers the host's genetic, clinical, and environmental factors that shape the course of TB infection and disease.
Over a three-year period, a prospective, multicenter TB cohort will be established at several sites of the German Center for Infection Research (DZIF). The cohort will include patients with pulmonary TB (PTB) and extrapulmonary TB (ePTB), including drug-sensitive and drug-resistant cases. In addition to comprehensive clinical data, biological samples will be collected and stored in a central biobank.
The main goal of the project is a comprehensive phenotypic characterization of TB patients using modern diagnostic techniques such as imaging, lung and organ-specific function measurements, and laboratory-based diagnostics. This will create a unique clinical dataset that goes beyond the currently available data from high-incidence countries, where clinical and diagnostic capacities are often limited.
This data will be used to characterize TB phenotypes and analyze risk factors, including those for TB sequelae.


Pediatric Diagnostic Cohort
Diagnosing pediatric tuberculosis is highly important due to challenges posed by nonspecific symptoms. Timely diagnosis enables prompt treatment, reducing morbidity and mortality risks. Prioritizing innovation in pediatric TB diagnostics is essential to address these factors. Tailored diagnostic tools can enhance accuracy, speed, and accessibility of TB diagnosis in children, improving their treatment outcomes. Innovations like collecting bacilli in the breath or using stool and urine to enhance the diagnostic confirmation in children can improve detection and outcomes. Using next generation sequencing for drug susceptibility testing can help in adapting the treatment to the resistance pattern of each child. Finally, following children with TB and analyzing the changes in lung function testing before and after TB treatment can bring much needed knowledge on post TB lung disease in children.
Therapeutic Drug Monitoring (TDM)
This project investigates the potential for using therapeutic drug monitoring (TDM) in tuberculosis (TB) treatment at the Eastern European Study Site (EESS) of the German Center for Infection Research (DZIF) in Romania. The focus is on surveying existing structures and testing practical strategies that enable a stepwise and locally adapted introduction of TDM. First, a comprehensive overview of the current TDM landscape in Romania will be compiled. This includes the systematic identification of laboratories offering TDM for TB medications, including available analytes, costs, sample logistics, and transport routes. This inventory will serve to highlight existing capacities and identify potential regional partners. In parallel, cross-institutional standard operating procedures (SOPs) will be developed in collaboration with Romanian and international partners to harmonize workflows and standardize quality standards. In addition, web-based pharmacokinetic models will be tested using data from the DZIF EESS TB cohort to evaluate the models for the respective target group. Finally, selected TB cases will be discussed in an international expert forum to obtain clinical recommendations, exchange experiences, and sustainably strengthen local expertise.
Risk Score for TB-Associated Mortality
As one of the research projects at the Eastern European Study Site, a clinical risk score to predict mortality in tuberculosis is being developed and validated. The aim is to enable early risk stratification of patients at the time of TB diagnosis based on readily available clinical variables. Conceptually, the approach is modeled on established respiratory risk scores such as the CRB-65 score and transfers this principle to the TB context. The goal is to develop a pragmatic, easy-to-use tool that can be applied particularly in routine clinical practice and in high-burden, resource-limited settings. By enabling early identification of high-risk patients, the score is intended to support clinical decision-making, facilitate targeted intensification of care, and ultimately reduce TB-associated mortality in the region.
Implementation and evaluation of the targeted Next-Generation Sequencing (tNGS) Assay ‘FreezeTB’ for drug-resistant tuberculosis in the Republic of Moldova
By combining speed, affordability, and comprehensive resistance profiling, FreezeTB has the potential to significantly improve molecular drug susceptibility testing (DST) and support individualized treatment of multidrug-resistant and rifampicin-resistant tuberculosis (MDR/RR-TB).
This study aims to establish and evaluate the FreezeTB assay in the Republic of Moldova, a high-priority setting for TB control. The primary objective is to implement FreezeTB in the national TB reference laboratory and assess its performance for detecting resistance to first-line, second-line, and new or repurposed anti-TB drugs. The study will compare FreezeTB results with routine diagnostic methods, including Xpert® MTB/RIF Ultra, Xpert® MTB/XDR, and phenotypic DST, as well as with Illumina-based commercial tNGS platforms. In addition, operational feasibility—including turnaround time, hands-on time, and failure rates—will be evaluated. The generated evidence will support informed decisions on integrating FreezeTB into national TB diagnostic algorithms.
As part of the collaboration with the Nicolae Testemitanu State University of Medicine and Pharmacy and the Chiril Draganiuc Phthisopneumology Institute, the feasibility and clinical utility of targeted next-generation sequencing (tNGS) for the detection of drug resistance in multidrug-resistant and rifampicin-resistant tuberculosis (MDR/RR-TB) is being investigated. While existing rapid molecular tests cannot reliably detect resistance to essential drugs such as bedaquiline, linezolid, and pretomanid, phenotypic drug susceptibility testing (pDST) is associated with long waiting times, significantly delaying the early initiation of effective therapy. tNGS offers a solution by delivering comprehensive resistance profiles within just two days, thus enabling rapid, precise, and personalized treatment decisions. This study will compare the resistance profiles of MDR/RR-TB patients using tNGS and pDST to evaluate the diagnostic accuracy, applicability, and benefits of tNGS. By validating tNGS as a practical and effective diagnostic tool, the project will provide fundamental data for the integration of this technology into routine TB diagnostics, thus closing existing gaps in current testing methods. Expected outcomes include faster and more precise resistance determination, optimized treatment outcomes for patients, and valuable epidemiological insights contributing to global TB control.
The Research Center Borstel – Leibniz Lung Center, V. N. Karazin Kharkiv National University (Ukraine), and additional Ukrainian institutions are partners in the clinical partnership BREATHE. The initiative aims to sustainably strengthen medical education and clinical capacities in respiratory and infectious diseases in Ukraine during the ongoing war.
Since the beginning of the Russian invasion, Ukraine’s higher education and healthcare systems have been under immense pressure. Medical education has been severely disrupted, while at the same time the burden of communicable and non-communicable diseases has increased. BREATHE addresses these challenges by promoting medical education, stabilizing clinical structures, and supporting healthcare professionals working under wartime conditions.
The project is implemented within the framework of the Hospital Partnerships funding program by the Deutsche Gesellschaft für Internationale Zusammenarbeit (GIZ) and is financed by the German Federal Ministry for Economic Cooperation and Development (BMZ) and the Else Kröner-Fresenius Foundation (EKFS).
BREATHE includes an expanded online lecture series on pulmonology and infectious diseases, weekly international multidisciplinary case discussions on complex tuberculosis (TB) and non-tuberculous mycobacterial (NTM) cases, an annual advanced clinical training course in Ukraine, and regular training opportunities for Ukrainian physicians as part of international courses in Borstel. The partnership promotes continuous knowledge exchange, ensures patient care, and contributes to the long-term resilience and recovery of Ukraine’s healthcare system.
UNITE4TB (academia and industry united innovation and treatment for tuberculosis) is a public-private partnership consisting of academic institutions, small and medium-sized enterprises (SMEs), public organizations and pharmaceutical companies.
Over a period of seven years (2021-2028), the consortium will be active in around 40 trial sites on four continents (Europe, Asia, Africa and South America) to conduct novel phase 2 clinical trials. These are intended to help accelerate the development of new TB drugs and therapies.
With a financial volume of 180 million euros, UNITE4TB is the largest study in the history of drug development for tuberculosis in history.
The project aims to develop better tolerated drug regimens with shorter treatment duration that can be used to combat tuberculosis with different drug resistance patterns and comorbidities.
Through Professor Lange, the Research Center Borstel is represented in the 4-member steering committee of UNITE4TB. Ms. Ohanna Kirakosyan from the Clinical Infectiology Research Group coordinates the study centers of the consortium in Europe and Mr. Collins Musia is co-leader of the work package on biomarkers. Ms. Nika Zielinski is involved in data analysis and biomarker evaluation.
Further information can be found at: https://www.unite4tb.org/
TBnet e.V. is a German non-governmental organization (NGO) with offices in Riga, Latvia and at the Research Center Borstel. TBnet promotes clinically oriented tuberculosis research in Europe through the exchange and development of ideas and research protocols. It is a European research network that brings together clinicians and researchers in the fight against tuberculosis and aims to improve the quality of care for tuberculosis patients by addressing health inequalities, conducting multicenter clinical trials and training European clinicians and scientists.
In recent years, TBnet has made important contributions to tuberculosis research in the fields of epidemiology, prevention, diagnostics and therapy. A list of TBnet publications can be found here.
TBnet currently has over 640 active members from more than 50 countries (as of May 2024). Ongoing studies focus on improving methods for diagnosis and therapy monitoring, among other things.
Specifically, this involves urinary metabolome analyses, the evaluation of a miniMDR TB test and the improvement of the diagnostic quality of the interferon-gamma release assay (IGRA) in immunosuppressed patients. More general studies are concerned with TB management in organ transplant patients or with the question of the extent to which drug levels in the blood influence specific therapy markers.
Training includes the TBnet Academy, which is aimed at young scientists and doctors to provide them with comprehensive knowledge. In recent years, the TBnet Academy has been held in Athens, Tbilisi and Riga.
TBnet member meetings take place annually on the friday before the ERS conference, where there is an opportunity to exchange ideas, present and discuss the status of projects and initiate new research.
Membership is open to anyone who wishes to make a charitable contribution to tuberculosis research. TBnet does not charge a membership fee
One branch of TBnet is the Nontuberculous Mycobacteria Network (NTMnet). Originally, the Pediatric Tuberculosis Network (pTBnet) was also part of TBnet, but is now independent.
This network emerged in 2011 from the Clinical Infectiology Department of the Research Center Borstel. The current chair is held by Dr. Liga Kuksa from Riga in Latvia. The TBnet secretariat at the Research Center Borstel is headed by Anne Oleischeck (aoleischeck@fz-borstel.de).
This project explores the use of an educational escape room as an innovative teaching method within the TBnet Academy, an international and multidisciplinary training program focused on diagnosis, treatment and management of TB and NTM diseases. Escape rooms combine problem-solving, teamwork, and realistic clinical scenarios to actively engage participants and support meaningful learning beyond traditional lecture-based formats.
The study evaluates the acceptance, feasibility, and perceived educational value of a TB-specific escape room involving learners and faculty from different countries and professional backgrounds. Using a qualitative multicenter design, participants are invited to share their experiences through semi-structured online interviews. The analysis focuses on engagement, knowledge integration, confidence in TB concepts, and the development of teamwork and communication skills.
By capturing participant perspectives, this project aims to support educators and institutions in reflecting on innovative teaching approaches. The findings will inform the future use of interactive, collaborative learning formats in international training and broader medical education initiatives.


Global migration has increased in recent decades due to wars, conflicts, persecution, human rights violations and natural disasters, but also due to work or study opportunities. The risk of tuberculosis among migrants depends on the reasons for migration, socio-economic status, the nature of the journey and the risk of tuberculosis in the transit country, as well as the healthcare in the country of origin and the host country.
Despite advancements in TB care for migrants and new treatment strategies, decisions about treatment are often based on expert opinion rather than clinical evidence.
This document summarizes the current state of knowledge on TB diagnosis, TB treatment strategies in migrants, MDR-TB in migrants and HIV/TB co-infection in migrants arriving in Europe. Studies were summarized by meta-analysis when appropriate, otherwise a narrative summary was used.
Based on a systematic review, consensus recommendations were made by experts on all aspects of migrant care.
Developing a REDCap (Research Electronic Data Capture) database for TBnet research projects is important in order to centralize the data collection and management process and offers customizable data entry forms, facilitating efficient and standardized data entry. Having an operational data collection and management system will provide TBnet with the opportunity of mutualizing research data from different projects and having a more powerful platform for proposals and publications.
DetecTB, a study on biomarker-based diagnostics for tuberculosis, investigates non-invasive diagnostic methods for identifying TB patients. The aim is to identify biomarkers from blood, urine, stool, and sputum that enable a reliable TB diagnosis, particularly in patients without sputum production or with extrapulmonary TB.
At the University Medical Center Hamburg-Eppendorf, patients with suspected TB and control subjects with other pulmonary infections are enrolled in the study. A single collection of various biomaterials is performed and analyzed using modern methods such as mRNA signatures, cellular immunology, PATHFAST-LAM, EclLAM, CRISPR-Cas, stool PCR, and Xpert MTB-HR. The feasibility and diagnostic value of these approaches are systematically evaluated.
The primary goal is to identify novel biomarkers or biomarker combinations that enable non-invasive, rapid, and accurate TB diagnosis. Secondary goals include comparisons between pulmonary and extrapulmonary TB, gender-specific differences, and associations with disease course and prognosis.This prospective, single-center observational study includes 120 patients whose participation offers no direct medical benefit but can contribute to the long-term development of improved TB diagnostics. Data and samples are stored pseudonymously and shared only in anonymized form. The results could help reduce invasive diagnostic procedures and enable earlier initiation of therapy.
As part of a multicenter, prospective research project, the role of a symptom diary in pulmonary infections caused by non-tuberculous mycobacteria (NTM) is being investigated. The study is conducted across numerous specialized centers throughout Europe and is embedded within the NTMnet network. The objective of the project is to systematically capture changes in symptoms over the course of disease and treatment and to assess the extent to which these changes correlate with microbiological and radiological findings. By using a standardized symptom diary, the study aims to evaluate whether patient-reported outcomes can serve as early markers of disease progression or treatment response. In the long term, the NTM Diary initiative seeks to improve monitoring of NTM diseases and to more strongly integrate patient-centered endpoints into clinical decision-making processes.
Immune checkpoints are regulatory pathways of the immune system that play a central role in controlling, modulating, and maintaining the immune response. They help prevent excessive immune reactions and ensure immunological homeostasis. Among the best-characterized immune checkpoint molecules are programmed cell death protein 1 (PD-1) and its ligand PD-L1, which are involved in regulating T-cell activation, proliferation, and effector function.
The PD-1/PD-L1 signaling pathways represent immunoregulatory mechanisms in chronic infectious diseases such as tuberculosis. Persistent antigen exposure can lead to increased expression of PD-1 and PD-L1, which is associated with functional changes in T cells and a diminished antimycobacterial immune response. Simultaneously, this immunoregulatory axis represents an important mechanism for limiting inflammatory tissue damage.The aim of this study is to investigate the immunological mechanisms of disease progression and to identify potential immunological biomarkers and therapeutic targets in the context of tuberculosis.
Post-tuberculosis lung disease is a common but often underestimated consequence of surviving tuberculosis. Worldwide, approximately 150 million people are tuberculosis survivors, many of whom suffer from long-term health problems resulting from lung damage caused by the disease. It is estimated that around 50% of survivors develop a permanent reduction in physical capacity and suffer from chronic respiratory diseases. This sequela poses a serious health challenge and can significantly impair quality of life. Unfortunately, PTLD is often underdiagnosed and undertreated.
Albania remains heavily affected by tuberculosis, and until now, the exact number of people suffering from PTLD in the country has not been systematically recorded. In collaboration with physicians from the National Hospital for Lung Diseases in Tirana, a prospective observational cohort study will examine 100 tuberculosis patients 6 to 30 months after the end of their tuberculosis treatment. The study will record impairments in lung function, exercise intolerance, and depressive symptoms, allowing for a more precise analysis of the prevalence of post-tuberculosis lung disease (PTLD) in Albania and the development of targeted measures to improve the quality of life for tuberculosis survivors. The ASTRA study is the first comprehensive investigation of PTLD in Albania.
The study's findings are not only significant for Albania but could also serve as a model for other countries facing similar challenges in post-tuberculosis care.

The aim of the TBpredict study is to evaluate novel molecular and immunological markers that enable an earlier and more precise assessment of treatment success in multidrug-resistant tuberculosis. These include MBLA and LAM measurements in sputum, as well as RNA sequencing in blood. For the first time, these methods are being tested simultaneously in this study, allowing for a direct comparison. In addition, lung function tests and physical performance parameters are being examined to predict performance decline following recovery from the infectious disease. Approximately 50% of tuberculosis patients are affected by post-tuberculosis disease. They experience persistent reduced physical capacity as a result of lung tissue damage caused by tuberculosis, even after the infectious disease has been overcome.
The Republic of Moldova is a high-incidence area for antibiotic-resistant tuberculosis worldwide. The project is part of a long-standing, close scientific collaboration between the Borstel Research Center, the Nicolae Testemitanu State University of Medicine and Pharmacy (USMF) in Chișinău, and the Institute of Pneumology in Chișinău.
The study will prospectively enroll 50 patients with rifampicin-resistant or multidrug-resistant tuberculosis (RR-/MDR-TB) in Chișinău. Extensive data will be collected at five time points during the early phase of TB therapy, as well as 12 and 18 months after the start of treatment.
The study on CRISPR-based detection of tuberculosis (TB) investigates the diagnostic value of an assay for detecting the mobile genetic element IS 6110 in blood. The gold standard for detecting TB is the culture of mycobacteria from sputum. This procedure is costly and time-consuming. Diagnosis is not possible in patients from whom sputum cannot be obtained, e.g., in children or in extrapulmonary TB. In cases of existing HIV coinfection, sputum often yields false-negative results. Detection using CRISPR-dependent fluorescence from blood samples is possible within a few hours. In initial studies, the test has demonstrated good diagnostic value even in cases of extrapulmonary TB, children, and HIV coinfection.
The primary objective of this study is to validate the test for sputum-free detection of tuberculosis. Using samples from biomarker studies, the assay's suitability for monitoring disease progression will be evaluated. Due to their properties, CRISPR-based detection methods have the potential to be implemented in point-of-care devices, so the validation of these assays could make a significant contribution to faster and decentralized tuberculosis diagnostics. Researchers at Tulane University (New Orleans, Louisiana) have developed a lab-in-tube device that enables the detection of cell-free tuberculosis DNA from sputum in 2 hours. An evaluation of the lab-in-tube device is planned for the near future.
As part of the collaboration with the Nicolae Testemitanu State University of Medicine and Pharmacy and the Chiril Draganiuc Phthisopneumology Institute, the feasibility and clinical utility of targeted next-generation sequencing (tNGS) for the detection of drug resistance in multidrug-resistant and rifampicin-resistant tuberculosis (MDR/RR-TB) is being investigated. While existing rapid molecular tests cannot reliably detect resistance to essential drugs such as bedaquiline, linezolid, and pretomanid, phenotypic drug susceptibility testing (pDST) is associated with long waiting times, significantly delaying the early initiation of effective therapy. tNGS offers a solution by delivering comprehensive resistance profiles within just two days, thus enabling rapid, precise, and personalized treatment decisions. This study will compare the resistance profiles of MDR/RR-TB patients using tNGS and pDST to evaluate the diagnostic accuracy, applicability, and benefits of tNGS. By validating tNGS as a practical and effective diagnostic tool, the project will provide fundamental data for the integration of this technology into routine TB diagnostics, thus closing existing gaps in current testing methods. Expected outcomes include faster and more precise resistance determination, optimized treatment outcomes for patients, and valuable epidemiological insights contributing to global TB control.
Drug-resistant (DR) tuberculosis (TB) cases pose a challenge in the care for TB patients and remain a significant epidemiological issue. Globally, rifampicin- and multidrug-resistant (RR/MDR) cases caused an estimated 160 000 deaths in 2022. In low-incidence countries and regions, foreign-born individuals are often more commonly affected by DR TB.
Ukraine is one of the countries in the European region with the highest incidence of tuberculosis (TB) and is also globally one of the countries with the highest burden of drug-resistant TB (1 - 3). Further, Ukraine has recently been affected by two unprecedented challenges to the national healthcare system with the COVID-19 pandemic and the outbreak of an armed conflict. The annually reported notification rates for infections caused by Mycobacterium tuberculosis across the European Union and the European Economic Area (EU/EEA) have been gradually declining in the last decades. In 2022, both the total TB notification rates (49.3 versus 8.0/ 100 000) and the proportion of DR TB among bacteriologically confirmed pulmonary cases (4.9% versus 28.3%) in the EU/EEA were substantially lower than the level reported in Ukraine. As of December 2023, about 5 million displaced Ukrainians had received temporary protection status in the countries of the EU/EEA. As a result of this substantial population size increase, the total number of notified TB cases in Ukrainians in the region rose almost four-fold in 2022. The proportion of the DR TB cases of Ukrainian origin amounted to almost 20% of all DR TB cases in the region and over 30% of the laboratory confirmed Ukrainian TB cases were affected by DR TB . In this context we aim to explore the DR TB epidemiology in Ukrainian and other foreign-born TB cases notified in the EU/EEA.
The OPAT-NTM project addresses the application and evaluation of so-called sOPAT (self-administered outpatient parenteral antimicrobial therapy) for infections with non-tuberculous mycobacteria (NTM). The focus is on the question of how practical and safe long-term intravenous antibiotic therapy can be outside of an inpatient setting.
We analyze the clinical data of 26 patients of the Borstel Lung Clinic with pulmonary and non-pulmonary infections caused by various mycobacterial species (including M. avium, M. chimaera, M. abscessus, and M. chelonae). A central goal is to describe the duration and composition of the antibiotic regimens used and to determine the reasons for treatment discontinuation or modification. In addition to efficacy, side effects, complications of the port system, and the management of adverse events are being investigated.
Overall, we aim to contribute to the evidence base for a treatment concept that enables patients to have greater autonomy while supporting the long-term treatment of difficult-to-treat infections.
Adverse events can make the treatment of tuberculosis more difficult and can be associated with a reduction in the patient's quality of life. The underlying mechanisms are not yet known for all drugs, meaning that adverse events cannot be completely prevented to date.
The aim of this project is therefore to identify and validate gene signatures using machine-learning algorithms to predict severe adverse events before the start of tuberculosis treatment. Transcriptome-based signatures are particularly suitable here. In addition, possible explanatory approaches for the development of severe adverse events are to be generated by identification and biological interpretation of genes and pathways. This would be a possible foundation for further research aimed at gaining a better understanding of the mechanismsand, if necessary, taking preventive countermeasures.
With the help of this work, it will hopefully be possible in the near future to identify an increased risk of serious adverse events before exposure to medication and thus make a contribution to personalised medicine in tuberculosis treatment.
In a subproject, the gene expression of suprabasin (SBSN) has already been identified as a potential biomarker for the prediction of severe linezolid-associated neuropathies.
DRAMATIC is a prospective, Duration-Randomized, Partially Blinded, Phase 2 Study. The objectives are
1) to demonstrate a defined relationship between duration and the proportion of successful outcomes,
2) to determine which durations of the oral experimental regimen can be expected to be as effective as the current validated (but recently de-prioritized) 9-11-month injectable-containing regimen and
3) to define the safety profile of the experimental regimen.
The study randomizes 220 participants, including adults (18 years of age or older) and children (12-17 years of age) with MDR-TB, to 16, 24, 32 or 40 weeks of treatment with a 5-drug oral experimental regimen (bedaquiline, delamanid, linezolid, levofloxacin and clofazimine).
Four experimental treatment durations allow the study to estimate the efficacy of multiple durations of treatment, maximizing the probability to identify the shortest effective experimental regimen. With this design it can be avoided that the duration selected is either too short (thus not effective enough) or too long (thus incurring excess toxicity). By following patients to observe relapse, important information for the optimal treatment duration in a large Phase 3 trial is gathered.
Many biological markers (including metabolic products, proteins, RNA in sputum, blood or urine) will be analyzed to identify new alternatives to traditional culture-based methods for determining the bactericidal activity of a drug or drug combination to predict treatment failure and relapse.
The study is conducted at trial centers in Philippines and Vietnam and is sponsored by the University of California (UCS), and Novartis, Otsuka and Pfizer as industry collaborators. It involves scientists from USA (Boston University, University of Colorado, UCS, Harvard), Philippines, Vietnam and Borstel.
If a new and more effective standard regimen comes into use by the time DRAMATIC has been completed, DRAMATIC will still be able to identify a duration of the experimental regimen that is non-inferior to the new standard of care. This will facilitate moving promptly to a confirmatory Phase 3 trial.
The diagnosis of tuberculosis poses a major challenge, especially in vulnerable patient groups such as children, the elderly, or HIV-infected individuals. The current gold standard for tuberculosis diagnosis is the cultural detection of Mycobacterium tuberculosis in sputum samples. However, this method requires patients to be able to produce sufficient sputum, which is often problematic, especially in children, the elderly, and immunocompromised patients. Lipoarabinomannan (LAM), which is released by Mycobacterium tuberculosis and can be detected in body fluids, offers the possibility of non-invasive and faster diagnosis.
The Electrochemiluminescence LAM Research Assay (EclLAM, MSD, Rockville, Maryland, USA) is a highly sensitive immunoassay that uses an ELISA-like platform with electronic luminescence detection to accurately quantify even the lowest concentrations of LAM.
The aim of this research project is to evaluate the diagnostic accuracy of LAM-based detection methods in urine samples for the diagnosis of TB.
In cooperation with the Clinical Pharmacology working group at the University of Hamburg and the Department of Pharmaceutical Biosciences at Uppsala University, we are developing an innovative tool for optimizing tuberculosis treatment with second-line drugs in the "AccessTDMetrics" project. The aim is to provide an easily accessible tool for pharmacokinetic modeling of the drugs
By measuring the concentration of antibiotics in patients' blood (Therapeutic Drug Monitoring, TDM), the dosage of medication can be adapted to the resistance of bacteria and the risk of side effects. This allows second-line drugs to be used more effectively and side effects to be avoided. However, there is currently no easily accessible tool that can interpret the measured concentrations using pharmacokinetic models and help doctors make dosing decisions.
With generous support from the “Schleswig-Holsteinische Gesellschaft zur Verhütung und Bekämpfung der Tuberkulose und der Lungenkrankheiten e.V.”, we can generate pharmacokinetic cohort data as part of the project and validate existing pharmacokinetic models using the data. The best models will then be integrated into the web application TDMx.eu at the University of Hamburg in order to support doctors more effectively in the dosing of tuberculosis drugs.


 At the Research Center Borstel, a new biomarker (TB22) was identified that enables the individualization of the duration of tuberculosis therapy. For this purpose, a signature was identified from RNA sequences in the peripheral blood of patients. For further validation, access to blood samples from very well characterized tuberculosis patients from Mozambique, Uganda and eSwatini is available through participation in an EU project.
At the Research Center Borstel, a new biomarker (TB22) was identified that enables the individualization of the duration of tuberculosis therapy. For this purpose, a signature was identified from RNA sequences in the peripheral blood of patients. For further validation, access to blood samples from very well characterized tuberculosis patients from Mozambique, Uganda and eSwatini is available through participation in an EU project.
The TB22 signature will be applied to transcriptome data to determine whether it can accurately diagnose tuberculosis in African children and PLHIV at the start of treatment. In addition, the dynamics of TB22 during treatment will be assessed to determine if the model can accurately predict the time at which the patient has achieved a relapse-free cure.
Transcriptome data may also enable the discovery of new RNA signatures that are not yet known, such as signatures to predict disease progression, detect active tuberculosis, distinguish between tuberculosis and non-tuberculous lung disease, monitor response to treatment, or predict tuberculosis relapse in early stages.
In a longitudinal cohort, samples were taken at various points during treatment to obtain DNA and epigenetic data, and clinical data on disease severity in the form of culture data and imaging procedures, treatment results, medication and any side effects were collected. These data will be used to determine whether and which TB-relevant RNA expressions identified in previous studies can be attributed to DNA mutations or epigenetic disorders in order to draw conclusions about supplementary epigenetic or immunomodulatory therapy. In addition, it will be investigated whether there is a correlation between epigenetic disorders and the clinical manifestations, therapy results and immune cell activity or with existing concepts of personalized TB therapy (e.g. endotypes). Furthermore, it will be investigated whether the epigenetic profile is altered by certain drugs used in TB therapy.
Antibiotic resistance is making the treatment of tuberculosis increasingly difficult. Cultural methods to determine antibiotic resistance are complex and time-consuming. It usually takes several months between the diagnosis of tuberculosis and the receipt of an antibiogram. New methods of gene sequencing allow rapid and comprehensive information on mutations in the genome of tuberculosis bacteria that are associated with antibiotic resistance. These molecular predictions of antibiotic resistance using targeted sequencing technologies (t-NGS) have been officially recommended by the WHO since summer 2023 for the prediction of antibiotic resistance and the composition of treatment regimens. The Research Center Borstel is significantly involved in this process and the preparation of the WHO recommendations.
Whether the treatment results of patients affected by multidrug-resistant tuberculosis (MDR-TB) can actually be improved by using the new technologies is plausible, but has never been proven in a clinical study.
The Research Center Borstel has entered into a strategic partnership with the University of Cape Town, South Africa, to jointly address these and other questions. Our research group is supporting colleagues from the University of Cape Town (Prof. Dr. Keertan Dheda, MD PhD) in the T3 Study 2024, which is investigating the impact of tNGS technology on treatment outcomes of patients with MDR-TB in South Africa. The first patients were enrolled in spring 2024.
The overall endpoint of the study is to determine the impact of t-NGS information-based treatment regimens on treatment outcomes of patients with MDR-TB.
Phase 1 primary endpoint:
proportion of patients initiated on treatment with ≥4 likely effective drugs within 14 days of diagnosis of rifampicin-resistant TB in each group (n=120).
Phase 2 primary endpoint:
rate of unfavorable outcomes 6 months after treatment initiation in each group (n=240 including phase 1 patients)
Phase 3 primary endpoint:
rate of unfavorable outcomes in each group 12 months after treatment initiation (n=300 including phase 2 patients).
The Xpert MTB/RIF Ultra is a rapid molecular assay in cartridge format, first introduced in 2017 as an upgrade of the Xpert MTB/RIF assay for diagnosing tuberculosis. While trace-positive results may represent detection of residual DNA from prior TB, sample cross-contamination, or laboratory error, they may also represent opportunities to detect active TB disease at an early stage. Stool is a clinical sample recommended by the WHO for the diagnosis of TB in children and gaining increasing evidence for adults. We are analyzing trace results from stool in a case-control study of TB patients paired with healthy controls.
A transcriptome model has been developed at the Borstel Research Centre to monitor treatment response in patients with pulmonary tuberculosis. The model uses the response of the human body to an infection with tuberculous mycobacteria to determine whether someone is infected. It could also be used to determine very precisely when someone has been treated with antibiotics long enough to be cured. This means that treatment durations could one day be adapted to each patient and overall shortened.
The model, which is based on 22 genes (TB22 for short), can also be used for diagnostics. This is particularly interesting for children, as many laboratory methods fail to detect tuberculosis in children. There is then no clear evidence of M. tuberculosis, so the diagnosis can only be made clinically. The aim of this research project is to test whether the TB22 model can help in the diagnosis of children or people with HIV infection. In close collaboration with Baylor College of Medicine, Houston, USA, we have been able to obtain RNA samples from children with tuberculosis in Eswatini in southern Africa. The first samples are currently on their way to Borstel and we are starting the measurements for the TB22 transcriptome model.
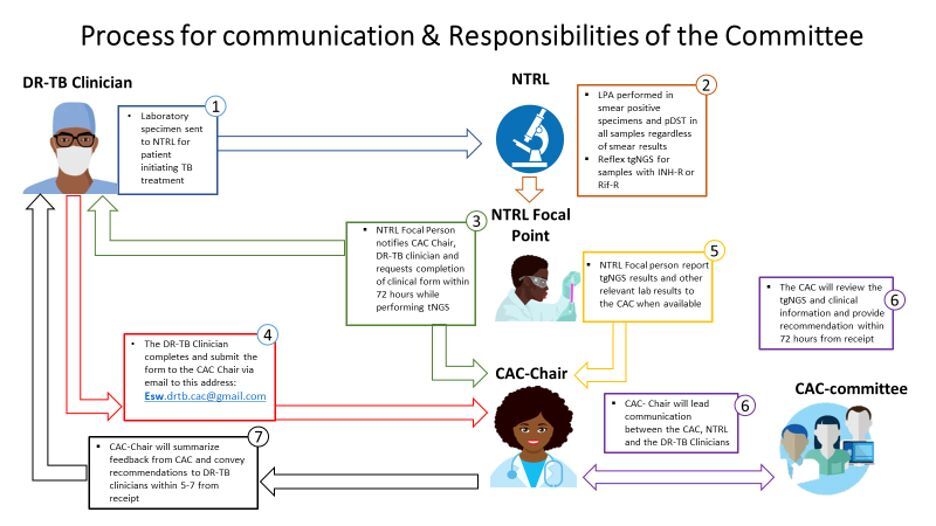
National TB Drug Resistance Surveys conducted in Eswatini in 2009/2010 and 2018 revealed transmission of a rifampicin resistant (RR) Mycobacterium tuberculosis complex (Mtbc) strain harboring the rpoB I491F mutation. This strain increased in prevalence from 30 % in the 2009/2010 survey to 58% in the 2018 survey. Current commercial molecular rapid diagnostics (MRD) and phenotypic drug susceptibility testing (pDST) by MGIT do not detect RR caused by this mutation, leading to a diagnostic gap and suboptimal TB treatment. In response, the Eswatini National Tuberculosis Program in collaboration with the Baylor College of Medicine Global TB Program and other key partners implemented a pilot project, utilizing targeted next generation sequencing (tNGS) for molecular drug susceptibility testing (mDST) of clinical Mtb strains from November 2021 to December 2022. A Clinical Advisory Committee (CAC) with clinical, laboratory and public health expertise was formed to guide optimization of treatment for patients with additional tNGS results.
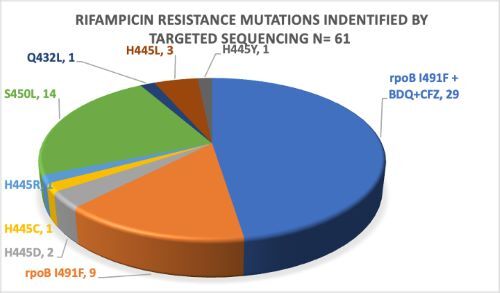
During the period of November 2021-December 2022, a total of 85 samples were sequenced and 61 RR strains were identified. Out of these, 38(62%) had rpoB I491F mutation with 29 (76%) of the rpoB I491F strains having an additional resistance to Bedaquiline and Clofazimine. From these results, 40 patients were followed up and their clinical reports were submitted to the CAC for treatment guidance.
Without using tNGS in DR-TB diagnosis in Eswatini, 38/61(62%) of MDR/RR-TB with rpoB I491F mutation and 29/38(76%) with additional Bdq and Cfz resistance would be missed. Based on tNGS results and CAC recommendations, 14/40(35%) of the patients benefitted from optimized treatment regimens. With treatment adaptation, 10/14(71%) of patients with worsening clinical condition on initial regimen (“treatment failure”) achieved successful treatment outcomes.
Detection of the I491F rpoB mutation and drug resistance mutations for new and repurposed drugs should be considered when developing MRDs. Newer medicines are needed, as bedaquiline resistance is becoming more common.
For more information, please contact the Eswatini Health Laboratory services Chief Laboratory Technologist, Mrs. Sindisiwe Dlamini at sindydlamini36@yahoo.com
The Baylor College of Medicine Global TB Program’s collaborative HIV/AIDS training program, Siyakhula (meaning “we are growing” in siSwati): Growing HIV/TB Research Knowledge for Growing Healthy Kids in Eswatini, seeks to bolster research capacity in Eswatini focused on HIV/AIDS and TB in pediatric populations. We seek to accomplish this goal by:
- Capitalizing on existing pediatric HIV expertise to enhance the investigation of children and adolescents impacted by HIV/TB and to provide long-term training that will produce independent Swazi investigators skilled in the most recent advances in the fields of epidemiology; biostatistics and bioinformatics; behavioral science and health promotion; and health systems management and policy.
- Providing intensive in-country training in Child Health Applied Research Training (CHART) to well-qualified Siyakhula scholars from partner institutions in Eswatini.
- Supporting local research infrastructure and the existing research community while enriching the environment and capacity to lead educational research opportunities for future health professionals.
Siyakhula is a blended binational approach, primarily driven by Eswatini-based learning forums, which are augmented by strategic U.S.-based learning opportunities. Two supplemental projects expand the program's rigor by integrating expertise on the intersection of climate change and public health, and the impact of premature ageing caused by HIV/TB and associated co-morbidities.
Two of the three doctoral scholars (independent investigators) are progressing successfully through their PhD programs, having completed two years of study. The third doctoral candidate will begin their studies in August 2024.
Siyakhula trained the first cohort of 15 CHART scholars in 2022 and continues to promote their growth and education through unique eSwatini-based learning activities, including supporting eight scholars to design, implement, and analyze independently developed research projects. Siyakhula is currently training its second cohort of 25 scholars. Under the leadership of Dr. Anna Mandalakas, the project PI, a robust group of multinational lecturers and mentos including partners from Research Center Borstal provide a rich collection of lectures.
For more information, please contact the Siyakhula Program Director, Dr. Debrah Vambe at debrah.vambe@bcm.edu.

The Integrated Network of Scholars in Global Health Research Training (INSIGHT) consortium comprises four U.S. academic institutions (Baylor College of Medicine’s Global TB Program, University of Alabama at Birmingham, University of Maryland Baltimore, University of Pittsburgh) and their affiliated international institutions in Africa, Asia, and the Americas. The program is sponsored by the Fogarty International Center (FIC) and several collaborating Institutes and Centers at the U.S. National Institutes of Health (NIH) and is one of the seven consortia in the NIH Fogarty Launching Future Leaders in Global Health (LAUNCH) Research Training Program. INSIGHT aims to:
- Create collaborative, multidisciplinary global health research that integrates cross institutional pairing of mentors
- Provide one year of mentored research training for 105 scholars
- Promote and support diversity, equity, and inclusion in global health research training
- Support the transition of scholars into successful and sustainable research careers
This opportunity supports mentored research training in global health for eligible U.S. doctoral candidates and U.S. and LMIC postdoctoral (MD, MD/PhD, ScD or equivalent degree) fellows. Successful applicants spend 12 months abroad at one of 27 affiliated institutions across 20 countries, where they gain experience conducting research in international settings. LMIC postdocs have short-term training at the affiliated U.S. institution. Trainee research projects are supported by experienced and dedicated mentors with expertise in clinical, public health, laboratory, and implementation research.
More information on the INSIGHT training program can be found on the INSIGHT website.
Tuberculosis (TB) is the world’s leading infectious cause of mortality and responsible for one third of deaths in people living with human immunodeficiency virus (HIV). Children and adolescents living with HIV are disproportionately affected due to inadequate preventive services, large case detection gaps, treatment and adherence challenges, and knowledge gaps. The TB GAPS project, funded by the U.S. Centers for Disease Control and Prevention (CDC) and implemented by Baylor College of Medicine’s Global TB Program, in collaboration with partners such as Research Center Borstel, is generating evidence to inform interventions targeting several of these weaknesses in the TB/HIV cascade of care through:
- Assessing the performance of novel TB screening and diagnostic algorithms among children, adolescents, and adults living with HIV and presenting for routine care across a network of family-centered HIV clinics in five sub-Saharan African countries as compared to the current WHO recommended symptom based screening and diagnostic strategy.
- Comparing the proportion of people living with HIV (PLHIV) who initiate and complete TB preventive therapy (TPT) with different regimens within the context of a patient centered differentiated service delivery model allowing selection of TPT regimen and randomly providing enhanced adherence support versus the standard of care for TPT and adherence support.
- Evaluating the cost-effectiveness of successful TB screening and diagnostic strategies and TPT regimens among children, adolescents, and adults living with HIV.
- Disseminating and promoting uptake of evidence based best practices in TB prevention, detection, and treatment.
Under the direction of Dr. Mandalaks, project PI, The TB GAPS study began enrolling clients in July 2023 in Eswatini followed by Lesotho in October 2023, Malawi in January 2024, and Uganda in February 2024. The implementation team is in the process of initiating enrollment in Tanzania. The study is expected to run through early 2026.
More information about TB GAPS can be found at www.tbgaps.org.
DZIF (German Center for Infection Research)
- Individualization of the duration of TB treatment
- Identification of tuberculosis endotypes
- DZIF MD Projects:
- Development of a tuberculosis therapeutic drug monitoring platform
- Molecular prediction of treatment regimens in drug -resistant tuberculosis
- Long-term treatment outcomes in patients with multidrug-resistant tuberculosis
- The impact of migration related to the Russia-Ukraine conflict on the epidemiology of tuberculosis infections in the EU and the EEA
- Unmask-TB
- Detection of Mycobacterium tuberculosis from respiratory masks in adults with pulmonary tuberculosis
EU-Projects
- anTBiotic (EU-H2020)
- ClickTB: Novel Clinical Candidates for TB (EDCTP)
- Stool4TB (EDCTP)
TBnet Projects
- Tuberculosis risk after organ transplantation in Europe
- International consensus document on molecular prediction of drug resistance
- Use of hepatoprotective drugs as adjunctive therapy for tuberculosis in Europe
- Tuberculosis in foreign-born individuals in Europe
- Investigation of the "QuantiFERON-TB-Gold-Plus"-Test in immunodeficient individuals
- Management of XDR-Tuberculosis in Europe
Further projects:
- Epidemiology:
- Multidrug-resistant tuberculosis
- TB-Database Medical Clinic Borstel
- DR-TB analysis in migrants in the EU/EAA (partnership with ECDC)
- Prevention:
- Effectiveness of preventive antibiotic treatment depending on age, tuberculosis prevalence and Mycobacterium tuberculosis infection status
- Diagnostics:
- Sputum MBLA as a monitoring marker during pulmonary tuberculosis therapy
- Stool-based diagnosis of pulmonary tuberculosis
- Lipoarabinomannan (LAM) as a potential marker for tuberculosis treatment monitoring
- Lipoarabinomannan (LAM) as a potential marker for the diagnosis of tuberculosis from sputum
- Therapy:
- Clinical standards for antimicrobial stewardship in tuberculosis
- Complication rate of outpatient parenteral antimicrobial therapy of infection with Mycobacterium tuberculosis
- Changes in microbiota as a function of tuberculosis therapy
- Impact of bedaquiline on treatment outcome in drug-resistant tuberculosis
- Use of TNF antagonists and JAK inhibitors in patients with previous tuberculosis
- Consensus management recommendations for less common non-tuberculous mycobacterial pulmonary diseases
Background:
The World Health Organization recommends standardised treatment durations for patients with tuberculosis (TB). We identified and validated a host-RNA signature as a biomarker for individualised therapy durations for patients with drug-susceptible (DS)- and multidrug-resistant (MDR)-TB.
Methods:
Adult patients with pulmonary TB were prospectively enrolled into five independent cohorts in Germany and Romania. Clinical and microbiological data and whole blood for RNA transcriptomic analysis were collected at pre-defined time points throughout therapy. Treatment outcomes were ascertained by TBnet criteria (6-month culture status/1-year follow-up). A whole-blood RNA therapy-end model was developed in a multistep process involving a machine-learning algorithm to identify hypothetical individual end-of-treatment time points.
Results:
50 patients with DS-TB and 30 patients with MDR-TB were recruited in the German identification cohorts (DS-GIC and MDR-GIC, respectively); 28 patients with DS-TB and 32 patients with MDR-TB in the German validation cohorts (DS-GVC and MDR-GVC, respectively); and 52 patients with MDR-TB in the Romanian validation cohort (MDR-RVC). A 22-gene RNA model (TB22) that defined cure-associated end-of-therapy time points was derived from the DS- and MDR-GIC data. The TB22 model was superior to other published signatures to accurately predict clinical outcomes for patients in the DS-GVC (area under the curve 0.94, 95% CI 0.9-0.98) and suggests that cure may be achieved with shorter treatment durations for TB patients in the MDR-GIC (mean reduction 218.0 days, 34.2%; p<0.001), the MDR-GVC (mean reduction 211.0 days, 32.9%; p<0.001) and the MDR-RVC (mean reduction of 161.0 days, 23.4%; p=0.001).

Conclusion:
Biomarker-guided management may substantially shorten the duration of therapy for many patients with MDR-TB.
Trial registration: ClinicalTrials.gov NCT02597621.
 Background:
Background:
In vitro, animal model and clinical evidence suggests that tuberculosis is not a monomorphic disease, and that host response to tuberculosis is protean with multiple distinct molecular pathways and pathologies (endotypes). We applied unbiased clustering to identify separate tuberculosis endotypes with classifiable gene expression patterns and clinical outcomes.
Methods:
A cohort comprised of microarray gene expression data from microbiologically confirmed tuberculosis patients was used to identify putative endotypes. One microarray cohort with longitudinal clinical outcomes was reserved for validation, as were two RNA-sequencing (seq) cohorts. Finally, a separate cohort of tuberculosis patients with functional immune responses was evaluated to clarify stimulated from unstimulated immune responses.
Results:
A discovery cohort, including 435 tuberculosis patients and 533 asymptomatic controls, identified two tuberculosis endotypes. Endotype A is characterised by increased expression of genes related to inflammation and immunity and decreased metabolism and proliferation; in contrast, endotype B has increased activity of metabolism and proliferation pathways. An independent RNA-seq validation cohort, including 118 tuberculosis patients and 179 controls, validated the discovery results. Gene expression signatures for treatment failure were elevated in endotype A in the discovery cohort, and a separate validation cohort confirmed that endotype A patients had slower time to culture conversion, and a reduced cure rate. These observations suggest that endotypes reflect functional immunity, supported by the observation that tuberculosis patients with a hyperinflammatory endotype have less responsive cytokine production upon stimulation.
Conclusion:
These findings provide evidence that metabolic and immune profiling could inform optimisation of endotype-specific host-directed therapies for tuberculosis.
 Development of a tuberculosis therapeutic drug monitoring platform
Development of a tuberculosis therapeutic drug monitoring platform
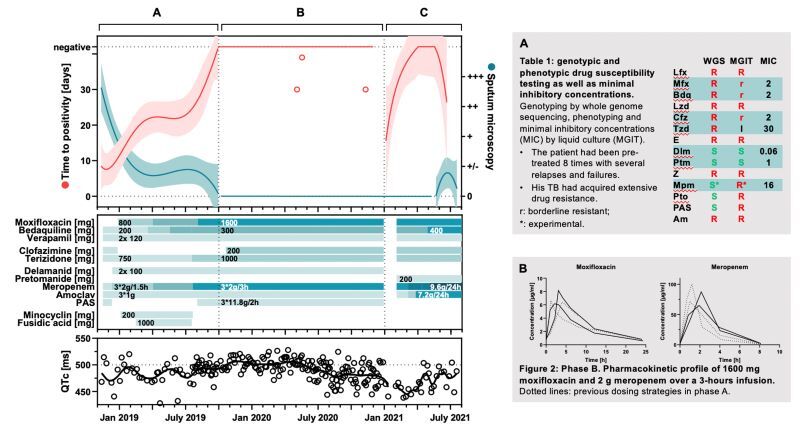
The treatment of drug-resistant Mycobacterium tuberculosis relies on complex antibiotic therapy. Inadequate antibiotic exposure can lead to treatment failure, acquired drug resistance, and an increased risk of adverse events. Therapeutic drug monitoring (TDM) can be used to optimize the antibiotic exposure. Therefore, we aimed to develop a single-run multiplex assay using high-performance liquid chromatography-mass spectrometry (HPLC-MS) for TDM of patients with multidrug-resistant, pre-extensively drug-resistant and extensively drug-resistant tuberculosis. A target profile for sufficient performance, based on the intended clinical application, was established and the assay was developed accordingly. Antibiotics were analyzed on a zwitterionic hydrophilic interaction liquid chromatography column and a triple quadrupole mass spectrometer using stable isotope-labeled internal standards. The assay was sufficiently sensitive to monitor drug concentrations over five half-lives for rifampicin, rifabutin, levofloxacin, moxifloxacin, bedaquiline, linezolid, clofazimine, terizidone/cycloserine, ethambutol, delamanid, pyrazinamide, meropenem, prothionamide, and para-amino salicylic acid (PAS). Accuracy and precision were sufficient to support clinical decision making (≤±15% in clinical samples and ±20-25% in spiked samples, with 80% of future measured concentrations predicted to fall within ±40% of nominal concentrations). The method was applied in the TDM of two patients with complex drug-resistant tuberculosis. All relevant antibiotics from their regimens could be quantified and high-dose therapy was initiated, followed by microbiological conversion. In conclusion, we developed a multiplex assay that enables TDM of the relevant first- and second-line anti-tuberculosis medicines in a single run and was able to show its applicability in TDM of two drug-resistant tuberculosis patients.
doi: 10.3390/pharmaceutics15112543
Molecular prediction of treatment regimens in drug -resistant tuberculosis
Background:
Comprehensive and reliable drug susceptibility testing (DST) is urgently needed to provide adequate treatment regimens for patients with multidrug-resistant/rifampicin-resistant tuberculosis (MDR/RR-TB). We determined whether next-generation sequencing (NGS) analysis of Mycobacterium tuberculosis complex isolates and genes implicated in drug resistance can guide the design of effective MDR/RR-TB treatment regimens.
Methods:
NGS-based genomic DST predictions of M. tuberculosis complex isolates from MDR/RR-TB patients admitted to a TB reference center in Germany between 1 January 2015 and 30 April 2019 were compared with phenotypic DST results of mycobacteria growth indicator tubes (MGIT). Standardized treatment algorithms were applied to design individualized therapies based on either genomic or phenotypic DST results, and discrepancies were further evaluated by determination of minimal inhibitory drug concentrations (MICs) using Sensititre MYCOTBI and UKMYC microtiter plates.
Results:
In 70 patients with MDR/RR-TB, agreement among 1048 pairwise comparisons of genomic and phenotypic DST was 86.3%; 76 (7.2%) results were discordant, and 68 (6.5%) could not be evaluated due to the presence of polymorphisms with yet unknown implications for drug resistance. Importantly, 549 of 561 (97.9%) predictions of drug susceptibility were phenotypically confirmed in MGIT, and 27 of 64 (42.2%) false-positive results were linked to previously described mutations mediating a low or moderate MIC increase. Virtually all drugs (99.0%) used in combination therapies that were inferred from genomic DST were confirmed to be susceptible by phenotypic DST.
Conclusions:
NGS-based genomic DST can reliably guide the design of effective MDR/RR-TB treatment regimens.
Long-term treatment outcomes in patients with multidrug-resistant tuberculosis
Objectives:
To describe long-term treatment outcomes in patients with multi-drug-resistant/rifampicin resistant tuberculosis (MDR/RR-TB) and validate established outcome definitions for MDR/RR-TB treatment.
Methods:
Among patients with MDR/RR-TB admitted to a German MDR/RR-TB referral centre from 1 September 2002 to 29 February 2020, we compared long-term treatment outcomes derived from individual patient follow-up with treatment outcomes defined by WHO-2013, WHO-2021 and the Tuberculosis Network European Trials Group-2016.

Results:
In a total of 163 patients (mean age, 35 years; standard deviation, 13 years; 14/163 [8.6%] living with HIV; 109/163 [66.9%] men, 149/163 [91.4%] migrating to Germany within 5 years), the treatment of culture-confirmed MDR/RR-TB was initiated. Additional drug resistance to a fluoroquinolone or a second-line injectable agent was present in 15 of the 163 (9.2%) Mycobacterium tuberculosis strains; resistance against both the drug classes was present in 29 of the 163 (17.8%) strains. The median duration of MDR/RR-TB treatment was 20 months (interquartile range, 19.3-21.6 months), with a medium of five active drugs included. The median follow-up time was 4 years (47.7 months; interquartile range, 21.7 65.8 months). Among the 163 patients, cure was achieved in 25 (15.3%), 82 (50.3%) and 95 (58.3%) patients according to the outcome definitions of WHO-2013, WHO-2021, and the Tuberculosis Network European Trials Group-2016, respectively. The lost to follow-up rate was 17 of 163 (10.4%). Death was more likely in patients living with HIV (hazard ratio, 4.28; 95% confidence interval, 1.26e12.86) and older patients (hazard ratio, 1.08; 95% confidence interval, 1.05e1.12; increment of 1 year). Overall, 101/163 (62.0%) patients experienced long-term, relapse-free cure; of those, 101/122 (82.8%) patients with a known status (not lost to-follow-up or transferred out) at follow-up.
Conclusion:
Under optimal management conditions leveraging individualized treatment regimens, long-term, relapse-free cure from MDR/RR-TB is substantially higher than cure rates defined by current treatment outcome definitions.
doi: 10.1016/j.cmi.2023.02.013
The impact of migration related to the Russia-Ukraine conflict on the epidemiology of tuberculosis infections in the European Union and the European Economic Area
The tuberculosis notification rate in Ukraine is around six times higher than the overall notification rate in the European Union (EU) and the European Economic Area (EEA). Ukraine is also a high priority country for the World Health Organization in terms of antibiotic-resistant tuberculosis. According to Eurostat, around 5 million Ukrainians have been displaced to EU and EEA countries since the beginning of the war between Russia and Ukraine.
In close collaboration with the European Center for Disease Prevention and Control (ECDC), we assessed the epidemiology of tuberculosis in cases of Ukrainian origin reported in the EU/EEA from 2019 to 2022 using routinely collected data.
Our results showed that the number of reported tuberculosis cases among Ukrainians in the region almost quadrupled in 2022 (n = 780, compared to an average of n = 201 in the three previous years). However, cases of Ukrainian origin continued to account for only a small proportion (2.2% in 2022) of all cases reported in the EU/EEA. In addition, notification rates among Ukrainian citizens in the EU/EEA were lower than those reported in Ukraine and remained below 20 per 100,000. The proportion of antibiotic-resistant forms of tuberculosis among cases of Ukrainian origin in the EU/EEA was high, reflecting the high proportion of resistant cases in Ukraine. In 2022, almost one in five antibiotic-resistant TB cases in the EU/EEA was of Ukrainian origin.
These results underline the importance of migrant-sensitive and patient-centered healthcare to ensure early presentation, initiation and continuation of treatment. Due to the high rate of antibiotic-resistant cases, pathogen detection and susceptibility testing in TB patients from Ukraine is crucial.
doi: 10.2807/1560-7917.ES.2024.29.12.2400094
Unmask-TB
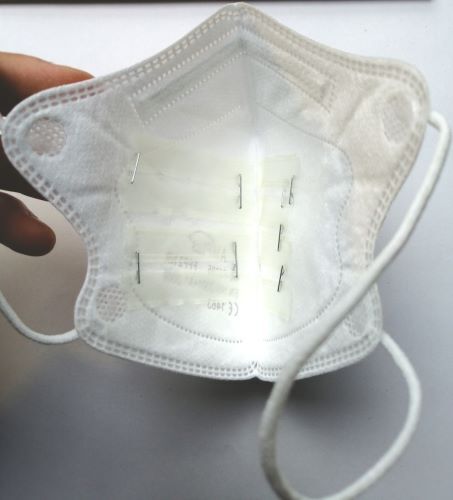
The aim of the Unmask-TB research project is to develop and test modified FFP2 masks as a diagnostic test for pulmonary tuberculosis in children. Even today, tuberculosis in children can often only be diagnosed clinically, i.e. there is no reliable detection of tuberculous mycobacteria in a laboratory test. In children, failure to recognize tuberculosis is dangerous; up to 40% of children under the age of five die of tuberculosis if undiagnosed and therefore untreated.
Tongue swabs, stool, urine, and the modified masks could be promising new sample types: Obtaining these samples is less burdensome for children compared to conventional methods, such as aspirating gastric liquid. Initial studies also indicate that some of these new methods can better detect tuberculosis in children. At the Borstel Research Centre, we have therefore adapted face masks that have been successfully tested on adults for children. These contain a 3D-printed polyvinyl alcohol strip that is designed to capture bacterial DNA in exhaled breath Our method is also part of the large ‘TB GAPS’ study conducted by Baylor College of Medicine, Houston, USA, in several countries in southern Africa. The German Centre for Infection Research has repeatedly provided us with extensive support in the development of the method.
The modified FFP2 masks for children had a low detection limit for M. tuberculosis-specific DNA in vitro. However, M. tuberculosis DNA was not detected in any of the 30 masks worn by children with pulmonary tuberculosis. FFP2 masks modified in this way may not be more effective at detecting M. tuberculosis in children than existing methods.

Detection of Mycobacterium tuberculosis from respiratory masks in adults with pulmonary tuberculosis
The aim of the study was to simplify the detection of Mycobacterium tuberculosis in adults and, if appropriate, to establish a less invasive and less costly method.
With a sensitivity of 86.5% in the original study (Williams et al. 2020), it seemed promising to continue investigating face mask sampling (FMS). In the original study, FFP2 masks with additional gelatine filters were used to detect MTB, later polyvinyl alcohol (PVA) strips proved more efficient. PVA strips were specially developed and produced at the Borstel Research Centre to ensure cost-effectiveness and comparability and can be produced locally at low cost in the future.
With an incidence of 80 new infections per 100,000 people, a third of which are MDR-TB, the Republic of Moldova is facing a major health crisis and offers an opportunity to continue our long-standing collaboration with the local health-care providers and diagnostic facilities.
In Moldova, we are working with the "Chiril Draganiuc" Lung Clinic and the National Reference Laboratory for Mycobacteria in Moldova to evaluate the efficacy of FMS in adults.
Between April 2024 and February 2025, a total of 117 adults were enrolled. 103 of these (88.0%) tested positive by sputum culture and/or Xpert MTB/RIF Ultra. Among participants testing positive by this combined reference standard, 59.2% (61/103) tested positive by FMS. Compared against sputum culture and sputum Xpert MTB/RIF Ultra, the sensitivity of FMS was 64.4% (95% CI: 54.4%-74.4%) and 58.3% (95% CI: 48.1%-68.0%), respectively. Among 90 participants with a positive sputum culture, FMS was positive in 6.0% (5/90) that were negative by sputum Xpert MTB/RIF Ultra.
These findings highlight the potential additive yield and complementary role of FMS. Where resources allow, FMS may serve as a valuable diagnostic tool used in parallel to conventional diagnostics to enhance the rapid detection of pulmonary TB in adults.



New tuberculosis treatments are needed to address drug resistance, lengthy treatment duration and adverse reactions of available agents. GSK3036656 (ganfeborole) is a first-in-class benzoxaborole inhibiting the Mycobacterium tuberculosis leucyl-tRNA synthetase. Here, in this phase 2a, single-center, open-label, randomized trial, we assessed early bactericidal activity (primary objective) and safety and pharmacokinetics (secondary objectives) of ganfeborole in participants with untreated, rifampicin-susceptible pulmonary tuberculosis. Overall, 75 males were treated with ganfeborole (1/5/15/30 mg) or standard of care (Rifafour e-275 or generic alternative) once daily for 14 days. We observed numerical reductions in daily sputum-derived colony-forming units from baseline in participants receiving 5, 15 and 30 mg once daily but not those receiving 1 mg ganfeborole. Adverse event rates were comparable across groups; all events were grade 1 or 2. In a participant subset, post hoc exploratory computational analysis of 18F-fluorodeoxyglucose positron emission tomography/computed tomography findings showed measurable treatment responses across several lesion types in those receiving ganfeborole 30 mg at day 14. Analysis of whole-blood transcriptional treatment response to ganfeborole 30 mg at day 14 revealed a strong association with neutrophil-dominated transcriptional modules. The demonstrated bactericidal activity and acceptable safety profile suggest that ganfeborole is a potential candidate for combination treatment of pulmonary tuberculosis.
As part of the EDCTP-funded "CLICK TB" project, new therapy regimes for the treatment of tuberculosis (TB), in particular drug-resistant TB (MDR-TB), are to be investigated. The aim is to clinically test new drug combinations using new active substances. The international consortium includes scientists from Spain and England (GSK), South Africa (TASK, UCT), Norway (University of Tromsø) and Borstel.
- Sanfetrinem cilexetil is a safe oral carbapenem with intrinsic stability against TB β-lactamases. The active substance has already been successfully clinically tested in a phase II study as a broad-spectrum antibiotic. This is the prerequisite for testing sanfetrinem in a phase IIa “Early Bactericidal Activity” study with regard to bactericidal activity in TB.
- GSK3036656 is the first compound in a new class of selective mycobacterial LeuRS inhibitors. The compound has successfully completed the Phase I study
The clinical studies are conducted at an experienced study center in South Africa (TASK) and include extensive biomarker testing (PET-CT and biological markers) to identify new alternatives to traditional culture-based methods for determining the bactericidal activity of a drug or drug combination. The biological biomarkers are measured and analyzed in the Clinical Infectiology laboratory. The tests include, for example, transcriptome analyses and the measurement of transrenal mycobacterial DNA. If the new drugs confirm the hopes, the study consortium aims to quickly transfer the drugs to a phase III study in order to make them available to TB patients and to make a contribution to the fight against TB.
The EDCTP funded project Stool4TB aims to evaluate an innovative stool-based qPCR diagnostic platform, under the hypothesis that it will narrow the extremely large Tuberculosis (TB) case detection gap by improving TB confirmation rates in children and people living with HIV (PLHIV).
Among children and PLHIV, TB continues to be a leading cause of morbidity and mortality. TB laboratory confirmation is particularly challenging in children and PLHIV given the difficulty in obtaining sputum samples and the paucibacillary nature of the disease.
Another target of Stool4TB is to create a TB diagnostic network among African high TB burden countries (Mozambique, eSwatini, and Uganda) with the capacity to conduct clinical studies of novel diagnostics and new drugs with a focus on children and PLHIV. Furthermore, a biobank of comparative specimens will be created.
As one of the project partners, the Research Center Borstel takes active part in establishing a biobank of samples for future TB-related research. Blood is collected into PAXgene and QuantiFERON-TB Gold (QFT) tubes with the specific goal to validate the TB22 signature and the concept of endotypes in TB in children. RNAseq and QFT data from the studies’ samples will be used to affirm TB22 potential to diagnose TB also in children, to prove its capacity to serve as a marker for outcome prediction in children, to show that it might be capable to serve as a marker for individualized therapy duration in children, to further validate the concept of endotypes in TB, and to perform multi-dimensional analysis in RNAseq and QFT data to further define endotypes in children. PAXgene tubes collected on sites will be shipped to Research Center Borstel where transcriptomic RNA analysis will be performed.
More information: https://www.stool4tb.org/
This European multicenter study shows that recipients of solid organ transplants (SOT) have a significantly increased risk of tuberculosis (TB), even in countries with low to moderate TB incidence. More than 5,800 adult transplant recipients from 15 centers across eight European countries were included in the study, conducted by researchers from the Tuberculosis Network European Trials Group (TBnet).
A total of 23 confirmed TB cases were observed, corresponding to an incidence of 68 cases per 100,000 person-years—approximately six times higher than in the general population. The risk was particularly high in Southern Europe, with 252 cases per 100,000 person-years, about nine times higher than in Central Europe. Patients with a migration background also had a significantly elevated risk of developing TB.
The study found that only one-third of transplant recipients were screened for latent TB before transplantation, and only one in ten received preventive therapy. Most TB cases occurred more than two years after transplantation, suggesting a combination of reactivation of latent infections and new infections.
The authors emphasize the importance of a regionally adapted prevention strategy: targeted screening in low-incidence regions and universal screening with preventive therapy in high-incidence regions could significantly reduce TB risk in immunocompromised transplant patients.
 Drug-resistant tuberculosis is a substantial health-care concern worldwide. Despite culture-based methods being considered the gold standard for drug susceptibility testing, molecular methods provide rapid information about the Mycobacterium tuberculosis mutations associated with resistance to anti-tuberculosis drugs. This consensus document was developed on the basis of a comprehensive literature search, by the TBnet and RESIST-TB networks, about reporting standards for the clinical use of molecular drug susceptibility testing. Review and the search for evidence included hand-searching journals and searching electronic databases. The panel identified studies that linked mutations in genomic regions of M. tuberculosis with treatment outcome data. Implementation of molecular testing for the prediction of drug resistance in M. tuberculosis is key. Detection of mutations in clinical isolates has implications for the clinical management of patients with multidrug-resistant or rifampicin-resistant tuberculosis, especially in situations when phenotypic drug susceptibility testing is not available. A multidisciplinary team including clinicians, microbiologists, and laboratory scientists reached a consensus on key questions relevant to molecular prediction of drug susceptibility or resistance to M. tuberculosis, and their implications for clinical practice. This consensus document should help clinicians in the management of patients with tuberculosis, providing guidance for the design of treatment regimens and optimising outcomes
Drug-resistant tuberculosis is a substantial health-care concern worldwide. Despite culture-based methods being considered the gold standard for drug susceptibility testing, molecular methods provide rapid information about the Mycobacterium tuberculosis mutations associated with resistance to anti-tuberculosis drugs. This consensus document was developed on the basis of a comprehensive literature search, by the TBnet and RESIST-TB networks, about reporting standards for the clinical use of molecular drug susceptibility testing. Review and the search for evidence included hand-searching journals and searching electronic databases. The panel identified studies that linked mutations in genomic regions of M. tuberculosis with treatment outcome data. Implementation of molecular testing for the prediction of drug resistance in M. tuberculosis is key. Detection of mutations in clinical isolates has implications for the clinical management of patients with multidrug-resistant or rifampicin-resistant tuberculosis, especially in situations when phenotypic drug susceptibility testing is not available. A multidisciplinary team including clinicians, microbiologists, and laboratory scientists reached a consensus on key questions relevant to molecular prediction of drug susceptibility or resistance to M. tuberculosis, and their implications for clinical practice. This consensus document should help clinicians in the management of patients with tuberculosis, providing guidance for the design of treatment regimens and optimising outcomes
Anecdotal information suggests that clinical practice regarding the use of putative hepatoprotective agents in tuberculosis treatment varies across countries in the WHO European Region. Between November 2023 and May 2024, we conducted a standardized questionnaire survey among representatives of the Tuberculosis Network European Trials Group (TBnet) in countries in the WHO European Region on the use of putative hepatoprotective agents in the treatment of tuberculosis patients.
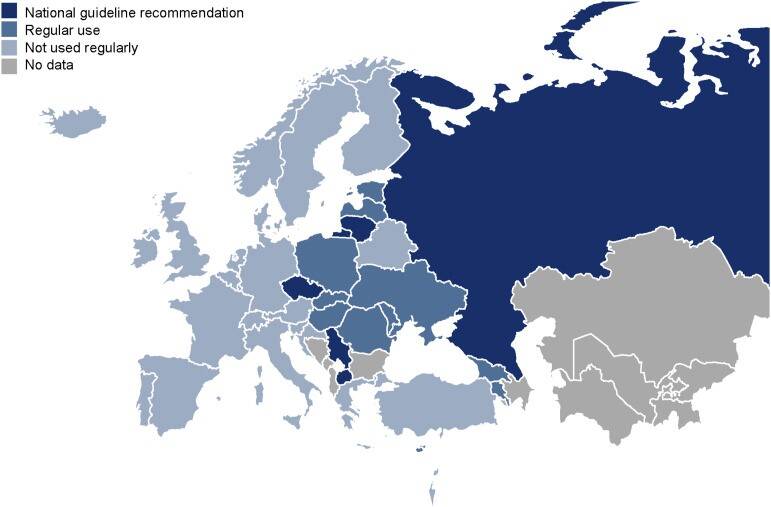 We received valid responses from 37 of 53 countries (69.8%), of which 16 (43.2%) reported regular use of putative hepatoprotective agents during TB treatment. Half of these countries (n = 8) are part of the former Soviet Union. In five countries, these agents are recommended in national guidelines. The most commonly used putative hepatoprotective agents were silibin/silymarin (n = 9, 56.3%), ursodeoxycholic acid (n = 5, 31.3%), and soy phospholipids (n = 4, 25.0%). Treatment duration varied: 56.3% (n = 9) took them for less than 1 month, 18.8% (n = 3) for 1-3 months, and 18.8% (n = 3) for 4-6 months. Putative hepatoprotective agents are widely used as an adjunct to tuberculosis treatment in the WHO European Region, particularly in the countries of the former Soviet Union, some of which have incorporated them into their national guidelines.
We received valid responses from 37 of 53 countries (69.8%), of which 16 (43.2%) reported regular use of putative hepatoprotective agents during TB treatment. Half of these countries (n = 8) are part of the former Soviet Union. In five countries, these agents are recommended in national guidelines. The most commonly used putative hepatoprotective agents were silibin/silymarin (n = 9, 56.3%), ursodeoxycholic acid (n = 5, 31.3%), and soy phospholipids (n = 4, 25.0%). Treatment duration varied: 56.3% (n = 9) took them for less than 1 month, 18.8% (n = 3) for 1-3 months, and 18.8% (n = 3) for 4-6 months. Putative hepatoprotective agents are widely used as an adjunct to tuberculosis treatment in the WHO European Region, particularly in the countries of the former Soviet Union, some of which have incorporated them into their national guidelines.
Background:
European-specific policies for tuberculosis (TB) elimination require identification of key populations that benefit from TB screening.
Aim:
We aimed to identify groups of foreign-born individuals residing in European countries that benefit most from targeted TB prevention screening.
Methods:
The Tuberculosis Network European Trials group (TBnet) collected, by cross-sectional survey, numbers of foreign-born TB patients residing in European Union (EU) countries, Iceland, Norway, Switzerland and the United Kingdom (UK) in 2020 from the 10 highest ranked countries of origin in terms of TB cases in each country of residence. Tuberculosis incidence rates (IRs) in countries of residence were compared with countries of origin.
Results:
Data on 9,116 foreign-born TB patients in 30 countries of residence were collected. Main countries of origin were Eritrea, India, Pakistan, Morocco, Romania and Somalia. Tuberculosis IRs were highest in patients of Eritrean and Somali origin in Greece and Malta (both > 1,000/100,000) and lowest among Ukrainian patients in Poland (3.6/100,000). They were mainly lower in countries of residence than countries of origin. However, IRs among Eritreans and Somalis in Greece and Malta were five times higher than in Eritrea and Somalia. Similarly, IRs among Eritreans in Germany, the Netherlands and the UK were four times higher than in Eritrea.
Conclusions:
Country of origin TB IR is an insufficient indicator when targeting foreign-born populations for active case finding or TB prevention policies in the countries covered here. Elimination strategies should be informed by regularly collected country-specific data to address rapidly changing epidemiology and associated risks.
In large parts of Europe, tuber culosis no longer plays a major role, although some people still carry the pathogen. However, for people with weakened immune systems, for example after an organ transplant or in the case of HIV infection, the bacterial infection can still be very dangerous. The QuantiFERON-TB Gold Plus (QFT+) test is often used as the standard diagnostic test. The QFT+ is an indirect test, meaning that it does not detect the pathogen itself, but rather the immune system's response to it. If the immune system is weakened, either deliberately to prevent rejection of a donor organ or by an immune system-weakening pathogen such as HIV, the immune response to the tuberculosis pathogen is also weaker. The QFT+ test may then produce false negative results more frequently.
culosis no longer plays a major role, although some people still carry the pathogen. However, for people with weakened immune systems, for example after an organ transplant or in the case of HIV infection, the bacterial infection can still be very dangerous. The QuantiFERON-TB Gold Plus (QFT+) test is often used as the standard diagnostic test. The QFT+ is an indirect test, meaning that it does not detect the pathogen itself, but rather the immune system's response to it. If the immune system is weakened, either deliberately to prevent rejection of a donor organ or by an immune system-weakening pathogen such as HIV, the immune response to the tuberculosis pathogen is also weaker. The QFT+ test may then produce false negative results more frequently.
In order to investigate the validity of the QFT+ test for detecting infection with mycobacteria and active tuberculosis, more than 2,600 patients were examined between 2015 and 2019 in a large-scale multicenter study conducted by TBnet. Additionally the patients were followed-up for two years to assess how well the test can determine the risk of developing tuberculosis. 1,788 people came from one of five groups with weakened immune systems: people who had undergone organ transplants, stem cell transplants, or who had rheumatoid arthritis, chronic renal failure, or HIV infection. A further 861 immunocompetent individuals served as a control group. The patients were treated at 21 medical centers in eleven European countries.
The study showed that the QFT+ test is not sufficiently reliable to be used on its own to diagnose active disease. It does not meet the WHO requirements for a tubercu losis test.
losis test.
In addition, the test's predictive power for future disease is very low. Even after two years, neither the positive nor the negative test subjects developed active tuberculosis- even if the QFT+ test was positive and no preventive therapy was administered. Only a few HIV-positive persons developed active tuberculosis in individual cases. The QFT+ test is therefore not sufficient to reliably predict the individual risk of tuberculosis in low-incidence countries. In future, additional risk factors – such as HIV status, immune status and origin – should therefore be given greater weight in the decision on preventive treatment.
In 2021, the World Health Organization changed the definitions for “extensively drug-resistant” tuberculosis (XDR-TB). In this form of the disease, the tuberculosis bacteria exhibit a particularlyhigh level of antibiotic resistance. In addition to the key drug rifampicin, there is also resistance to fluoroquinolones and bedaquiline and/orlinezolid. This form of tuberculosis is still very rare in Europe. 
In an observational retrospective cohort study, at many treatment centers in the WHO European Region data were collected from patients diagnosed with XDR-TB between 2017 and 2023. Of 11.003 patients with rifampicin-resistant tuberculosis, 188 patients (1,7%) from 16 countries had XDR-TB. Only four out of ten patients affected by XDR-TB achieved successful treatment outcomes, one out of ten died from the disease. Compared with other levels of drug resistance, treatment outcomes were significantly worse for XDR-TB. The findings oft he study highlight the need for adequate, individualised treatment regimens and optimised drug susceptibility testing.

Multidrug-resistant tuberculosis
Tuberculosis (TB) remains the foremost cause of death by an infectious disease globally. Multidrug-resistant or rifampicin-resistant TB (MDR/RR-TB; resistance to rifampicin and isoniazid, or rifampicin alone) is a burgeoning public health challenge in several parts of the world, and especially Eastern Europe, Russia, Asia and sub-Saharan Africa. Pre-extensively drug-resistant TB (pre-XDR-TB) refers to MDR/RR-TB that is also resistant to a fluoroquinolone, and extensively drug-resistant TB (XDR-TB) isolates are additionally resistant to other key drugs such as bedaquiline and/or linezolid. Collectively, these subgroups are referred to as drug-resistant TB (DR-TB). All forms of DR-TB can be as transmissible as rifampicin-susceptible TB; however, it is more difficult to diagnose, is associated with higher mortality and morbidity, and higher rates of post-TB lung damage. The various forms of DR-TB often consume >50% of national TB budgets despite comprising <5-10% of the total TB case-load. The past decade has seen a dramatic change in the DR-TB treatment landscape with the introduction of new diagnostics and therapeutic agents. However, there is limited guidance on understanding and managing various aspects of this complex entity, including the pathogenesis, transmission, diagnosis, management and prevention of MDR-TB and XDR-TB, especially at the primary care physician level.
doi: 10.1038/s41572-024-00504-2
TB-Database Medical Clinic Borstel
In the 10 years from 2012 to 2021, we have treated over 800 tuberculosis patients as inpatients, of which approx. 150 patients with multidrug-resistant tuberculosis (MDR-TB) and approx. 30 patients with extensively drug-resistant tuberculosis (XDR-TB). Data necessary for the correct diagnosis and successful treatment of individual patients was routinely collected in our clinic. All patient data was documented in accordance with the legal requirements of the Data Protection Regulation.
Data from the clinical laboratory, molecular biology or microbiology were recorded at close intervals in order to characterize changes in the quantity and antibiotic sensitivity of the tuberculosis bacteria and to document the course of the tuberculosis disease and the response to therapy. This enabled us to react quickly to any changes. In addition, information about the therapy (medication, dosage, operations if necessary), any side effects and the success of the therapy was recorded. In this way, we try to identify risk factors for delayed treatment response or treatment failure, among other things.
The data collected in everyday clinical practice was compiled in a database, greatly simplifying data collection for clinical studies and enabling additional scientific evaluations. The database was used to produce case studies on individual Borstel patients, e.g. on MDR silicotuberculosis in a quarry worker and on a patient with extremely resistant XDR tuberculosis. An interim evaluation of the database showed that tuberculosis bacteria survived significantly longer in active and former smokers than in kidney smokers.
The data from the database has been used in various completed and ongoing studies, e.g.
- Long-term treatment results for MDR tuberculosis
- MDR tuberculosis treatment based on DNA sequencing
- Port infections during intravenous outpatient therapy
- Prediction of linezolid side effects
- in the DZIF Biomarker Research project
- in the DZIF Therapeutic Drug Monitoring project
- in the Cluster of Excellence Precision Medicine: Individualized therapy of chronic lung infections
- in the TBnet study Management of XDR-TB in Europe
- in a global systematic review on treatment outcomes in children and adolescents with MDR tuberculosis (BENEFIT Kids)
DR-TB analysis in migrants in the EU/EAA (partnership with ECDC)
Tuberculosis cases with antibiotic resistance remain a significant epidemiological problem. Worldwide, rifampicin- and multidrug-resistant (RR/MDR) cases caused an estimated 160,000 deaths in 2022. In countries and regions with low tuberculosis incidence, foreign-born individuals are often more frequently affected by antibiotic-resistant tuberculosis.
In 2023, TB notifications among people of foreign origin in the European Union and the European Economic Area (EU/EEA) increased by 24.6% after decreasing between 2019 and 2020. The majority originated from African and East Mediterranean regions, with a 316.4% upsurge in TB among Ukrainians in 2022–23. Ukraine is one of the countries in Europe with the highest incidence of tuberculosis (TB) and also one of the countries worldwide with the highest burden of antibiotic-resistant TB. Furthermore, Ukraine has recently faced two unprecedented challenges to its national health system: the COVID-19 pandemic and the outbreak of an armed conflict. By December 2023, approximately 5 million displaced Ukrainians had been granted temporary protection status in EU/EEA countries. As a result of this substantial population increase, the total number of reported tuberculosis cases among Ukrainians in the region nearly quadrupled in 2022. Rifampicin-resistant/multidrug-resistant TB increased by ≥ 60% in 2019–23, mainly among Ukrainians.
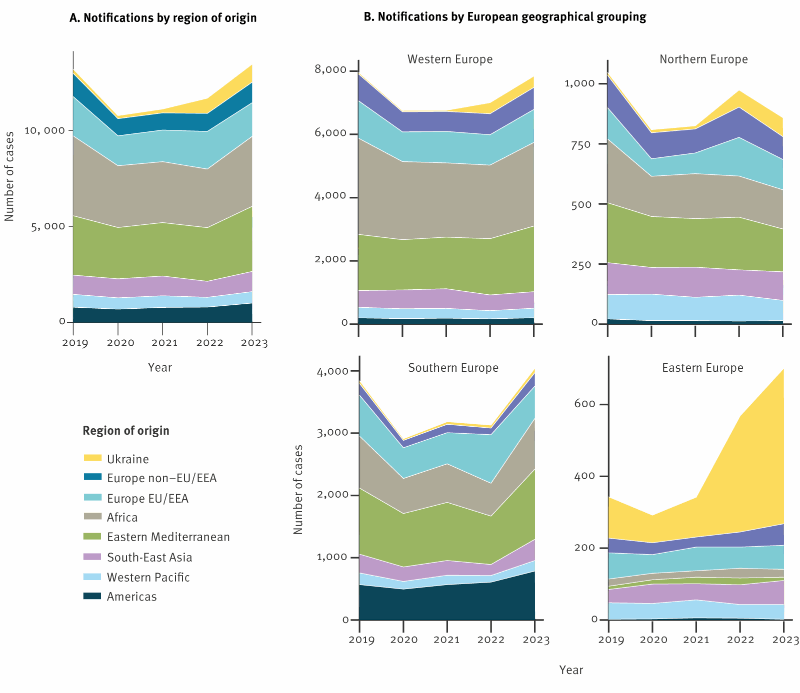
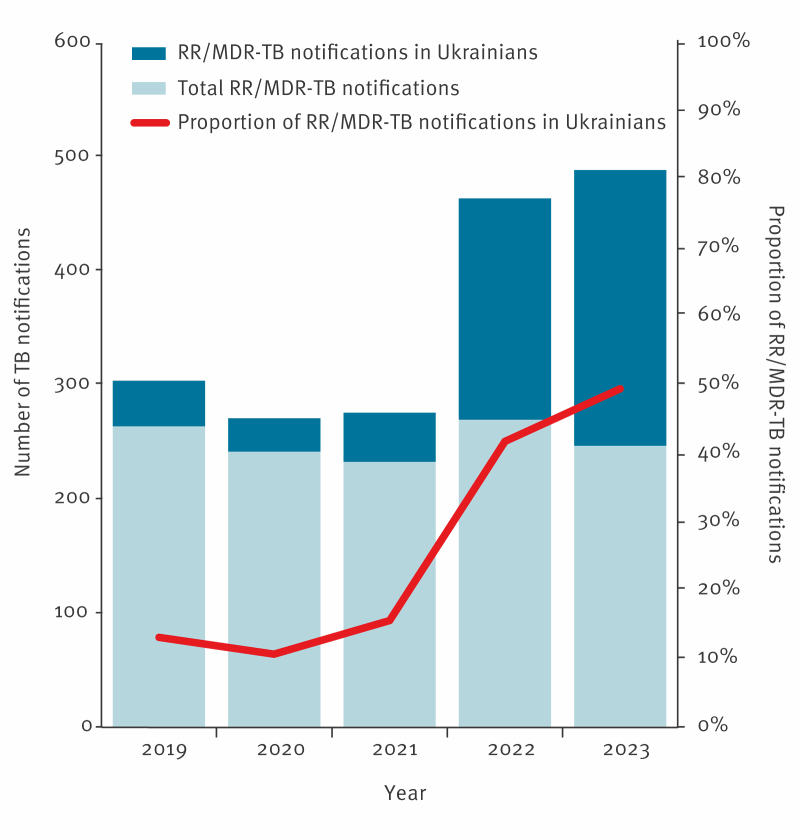
Rapid adaptation to shifting epidemiological patterns, increasing availability of drug susceptibility data, improved drug resistance surveillance, better follow-up of mobile populations and strengthening public health response is crucial to control TB spread within the EU/EEA.
Effectiveness of preventive antibiotic treatment depending on age, tuberculosis prevalence and Mycobacterium tuberculosis infection status
Tuberculosis is a preventable disease. However, there is debate regarding which individuals would benefit most from tuberculosis preventive treatment and whether these benefits vary in settings with a high burden and low burden of tuberculosis. We aimed to compare the effectiveness of tuberculosis preventive treatment in exposed individuals of differing ages and Mycobacterium tuberculosis infection status while considering tuberculosis burden of the settings.
In this systematic review and individual-participant meta-analysis, we investigated the development of incident tuberculosis in people closely exposed to individuals with tuberculosis. We searched for studies published between Jan 1, 1998, and April 6, 2018, in MEDLINE, Web of Science, BIOSIS, and Embase. We restricted our search to cohort studies; case-control studies and outbreak reports were excluded. Two reviewers evaluated titles, abstracts, and full text articles for eligibility. At each stage, two reviewers discussed discrepancies and re-evaluated articles until a consensus was reached. Individual-participant data and a pre-specified list of variables, including characteristics of the exposed contact, the index patient, and environmental characteristics, were requested from authors of all eligible studies; contacts exposed to a drug-resistant tuberculosis index patient were excluded. The primary study outcome was incident tuberculosis. We estimated adjusted hazard ratios (aHRs) for incident tuberculosis with mixed-effects Cox regression models with a study-level random effect. We estimated the number-needed-to-treat (NNT) to prevent one person developing tuberculosis. Propensity score matching procedures were used in all analyses. This study is registered with PROSPERO (CRD42018087022).
After screening 25 358 records for eligibility, 439 644 participants from 32 cohort studies were included in the individual-participantdata meta-analysis. Particip ants were followed for 1 396 413 person-years (median of 2·7 years [IQR 1·3–4.4]), during which 2496 people were diagnosed with incident tuberculosis. Overall, effectiveness of preventive treatment was 49% (aHR 0·51 [95% CI 0·44–0·60]). Participants with a positive tuberculin-skin-test (TST) or IFNγ release assay (IGRA) result at baseline benefitted from greater protection, regardless of age (0·09 [0·05–0·17] in children younger than 5 years, 0·20 [0·15–0·28] in individuals aged 5–17 years, and 0·17 [0·13–0·22] in adults aged 18 years and older). The effectiveness of preventive treatment was greater in high-burden (0·31 [0·23–0·40]) versus low-burden (0·58 [0·47–0·72]) settings. The NNT ranged from 9 to 34 depending on age among participants with a positive TST or IGRA in both high-burden and low-burden settings; among all contacts (regardless of TST or IGRA test result), the NNT ranged from 29 to 43 in high-burden settings and 213 to 455 in low-burden settings.
ants were followed for 1 396 413 person-years (median of 2·7 years [IQR 1·3–4.4]), during which 2496 people were diagnosed with incident tuberculosis. Overall, effectiveness of preventive treatment was 49% (aHR 0·51 [95% CI 0·44–0·60]). Participants with a positive tuberculin-skin-test (TST) or IFNγ release assay (IGRA) result at baseline benefitted from greater protection, regardless of age (0·09 [0·05–0·17] in children younger than 5 years, 0·20 [0·15–0·28] in individuals aged 5–17 years, and 0·17 [0·13–0·22] in adults aged 18 years and older). The effectiveness of preventive treatment was greater in high-burden (0·31 [0·23–0·40]) versus low-burden (0·58 [0·47–0·72]) settings. The NNT ranged from 9 to 34 depending on age among participants with a positive TST or IGRA in both high-burden and low-burden settings; among all contacts (regardless of TST or IGRA test result), the NNT ranged from 29 to 43 in high-burden settings and 213 to 455 in low-burden settings.
Our findings suggest that a risk-targeted strategy prioritising contacts with evidence of M. tuberculosis infection might be indicated in low-burden settings, and a broad approach including all contacts should be considered in high-burden settings. Preventive treatment was similarly effective among contacts of all ages.
Sputum MBLA as a monitoring marker during pulmonary tuberculosis therapy
Once tuberculosis has been diagnosed, anti-tuberculosis therapy is initiated and the course of treatment is monitored regularly over the next six months or more in order to detect treatment failure as early as possible. The most important proof of a treatment response is culture negation, i.e. the absence of growth of mycobacteria in microbiological culture over eight weeks. A molecular biological method for the rapid detection of Mycobacterium tuberculosis in sputum is the amplification of genomic mycobacterial DNA (e.g. GeneXpert®). However, this test system cannot distinguish between dead and viable TB bacteria, meaning that a positive result cannot reliably predict treatment failure. The determination of the expression of replicating genes in the new test system of the molecular tuberculosis bacterial load test (MBLA) has the advantage that only viable bacteria are detected.
In this prospective observational cohort study with Marit Neumann and Prof Dr Christoph Hölscher (Infection Immunology) the accuracy of TB-MBLA in assessing bacterial load was evaluated for the first time, to monitor treatment response in adults with pulmonary MDR/XDR tuberculosis. TB-MBLA results were compared with microscopy, the GeneXpert method and time to culture positivity (TTP) in Mycobacterial Growth Indicator Tube (MGIT) culture.
MBLA was superior in the early identification of successful culture conversion compared to microscopy and GeneXpert and could be a useful biomarker to evaluate novel entities in Phase IIA early-bactericidal-activity drug trials regardless of the degree of M. tuberculosis drug resistance.
doi: 10.1016/j.jinf.2024.106399
Stool-based diagnosis of pulmonary tuberculosis
Background:
Despite increasing availability of rapid molecular tests for the diagnosis of tuberculosis in high-burden settings, many people with tuberculosis are undiagnosed. Reliance on sputum as the primary specimen for tuberculosis diagnostics contributes to this diagnostic gap. We evaluated the diagnostic accuracy and additive yield of a novel stool quantitative PCR (qPCR) assay for the diagnosis of tuberculosis in three countries in Africa with high tuberculosis burdens.
Methods:
We undertook a prospective diagnostic accuracy study in Eswatini, Mozambique, and Tanzania from Sept 21, 2020, to Feb 2, 2023, to compare the diagnostic accuracy for tuberculosis of a novel stool qPCR test with the current diagnostic standard for Mycobacterium tuberculosis DNA detection from sputum and stool, Xpert-MTB/RIF Ultra (Xpert Ultra). Sputum, stool, and urine samples were provided by a cohort of participants, aged 10 years or older, diagnosed with tuberculosis. Participants with tuberculosis (cases) were enrolled within 72 h of treatment initiation for tuberculosis diagnosed clinically or following laboratory confirmation. Participants without tuberculosis (controls) consisted of household contacts of the cases who did not develop tuberculosis during a 6-month follow-up. The performance was compared with a robust composite microbiological reference standard (CMRS).

Findings:
The cohort of adolescents and adults (n=408) included 268 participants with confirmed or clinical tuberculosis (cases), 147 (55%) of whom were living with HIV, and 140 participants (controls) without tuberculosis. The sensitivity of the novel stool qPCR was 93·7% (95% CI 87·4-97·4) compared with participants with detectable growth on M tuberculosis culture, and 88·1% (81·3-93·0) compared with sputum Xpert Ultra. The stool qPCR had an equivalent sensitivity as sputum Xpert Ultra (94·8%, 89·1-98·1) compared with culture. Compared with the CMRS, the sensitivity of the stool qPCR was higher than the current standard for tuberculosis diagnostics on stool, Xpert Ultra (80·4%, 73·4-86·2 vs 73·5%, 66·0-80·1; p=0·025 on paired comparison). The qPCR also identified 17-21% additional tuberculosis cases compared to sputum Xpert Ultra or sputum culture. In controls without tuberculosis, the specificity of the stool qPCR was 96·9% (92·2-99·1).
Interpretation:
In this study, a novel qPCR for the diagnosis of tuberculosis from stool specimens had a higher accuracy in adolescents and adults than the current diagnostic PCR gold standard on stool, Xpert-MTB/RIF Ultra, and equivalent sensitivity to Xpert-MTB/RIF Ultra on sputum.
Funding:
National Institutes of Health (NIH) Allergy and Infectious Diseases and NIH Fogarty International Center.
doi: 10.1016/S2666-5247(23)00391-9
Lipoarabinomannan (LAM) as a potential marker for tuberculosis treatment monitoring
Lipoarabinomannan (LAM), a glycolipid found in the cell wall of mycobacteria, plays an essential role in the pathogenesis of tuberculosis (TB). LAM can be detected in bodily fluids such as urine, making it a suitable diagnostic biomarker, particularly in HIV-positive patients.
The current gold standard for TB diagnosis is the cultural detection of Mycobacterium tuberculosis in sputum samples. However, some patients are unable to produce sputum or produce insufficient amounts (e.g., children, the elderly, immunocompromised individuals). Various test systems aim to detect LAM in clinical samples to enable rapid and non-invasive diagnosis.
In this retrospective cohort study, the ultrasensitive electrochemiluminescent LAM test (EclLAM) from Meso Scale Discovery, Inc. was evaluated to measure LAM in the urine of HIV-seronegative patients with pulmonary TB, both for the diagnosis of active TB and for monitoring TB treatment. Samples obtained at various time points (before and after the start of therapy) from microbiologically confirmed TB patients in South Africa and Germany were analyzed.
Both antibody pairs used, FDX-01/FIND-28 and S4-20/A194-01, correctly identified patients with active TB. In addition, both antibody pairs correlated with the time to positive culture and were therefore suitable for therapy monitoring. Overall, these results underscore the potential of LAM as a biomarker for both diagnosis and treatment monitoring. Further research should prioritize the development of non-invasive, sensitive, specific, and quantitative LAM tests.
doi: 10.1016/j.tube.2025.102619
Lipoarabinomannan (LAM) as a potential marker for the diagnosis of tuberculosis from sputum
Despite modern therapies, tuberculosis (TB) remains one of the world's leading infectious diseases. A key diagnostic goal is to detect infections as early and reliably as possible. Lipoarabinomannan (LAM), a component of the mycobacterial cell wall, is detectable in various bodily fluids during an active infection.
The PATHFAST™ TB LAM Ag Test (PATHFAST LAM, PHC Corporation, Tokyo, Japan) is an automated in vitro diagnostic test for quantifying LAM. In this research project, sputum samples from 27 individuals with pulmonary tuberculosis and 20 control subjects were analyzed. The diagnostic accuracy of the test, the correlation between LAM concentration and bacterial load, and the reproducibility of the test results were evaluated.
LAM concentrations were higher in sputum samples from individuals with TB (median: 241.5 pg/mL) compared to those from individuals without TB (0 pg/mL), with a sensitivity of 85.2% and a specificity of 100%. LAM concentration correlated with both microscopy and smear findings (rho = 0.86) and with the time to positivity in MGIT culture (rho = −0.65). Sample homogenization improved the reproducibility of the test results (coefficient of variation 4.5% vs. 72.6%).The PATHFAST LAM meets the WHO Target Product Profile criteria for tuberculosis diagnosis from sputum. The LAM concentration correlates with the bacterial load, and the reproducibility of the test results benefits from homogenization of the samples.
doi: 10.1016/j.jinf.2025.106662
Clinical Standards for Antimicrobial Stewardship in Tuberculosis
This project aimed to develop clear, evidence-based recommendations for the responsible use of antituberculosis drugs. While antimicrobial stewardship (AMS) is gaining increasing importance in infectious diseases in general, it remained poorly defined in the field of tuberculosis – a significant shortcoming, particularly given that rifampicin-resistant TB (RR-TB) has been classified by the WHO as one of the four most critical bacterial threats. To close this gap, an international expert panel of around 60 specialists developed sound recommendations for AMS in TB treatment using a structured Delphi process. The goal of the clinical standards is to optimize the rational use of antituberculosis drugs in order to improve treatment quality, minimize the risk of resistant TB strains, and contribute to global TB control in the long term. The interdisciplinary collaboration of leading experts in TB diagnostics, therapy, and AMS from all regions of the world ensured that the developed standards are scientifically sound, practical, and tailored to the needs of health systems worldwide. This project represents an important step toward establishing AMS as an integral component of TB treatment and sustainably strengthening the global strategy against drug-resistant TB pathogens.
doi: 10.5588/ijtldopen.25.0522
Complication rate of outpatient parenteral antimicrobial therapy for infection with M. tuberculosis
The treatment of tuberculosis is becoming increasingly difficult due to increasing antibiotic resistance. For some patients with multidrug-resistant (MDR) or extensively drug-resistant (XDR) tuberculosis injectable antibiotics (meropenem and/or amikacin) have to be used. As the therapy lasts several months, intravenous therapy must be continued in an outpatient setting. A port is inserted and the patient is trained to administer the medication independently.
The study followed 89 patients with multidrug-resistant (MDR) and pre-extensively drug-resistant (pre-XDR) TB over a six-year period. Most feared complications of at-home intravenous therapy are infections or thrombosis of the indwelling catheter. They can lead to sepsis and require surgical removal of the catheter. However, only 8 patients suffered from these severe complications in the study period, with an overall complication rate of just 0.3 per 1,000 treatment days. None of these complications was fatal. Adverse drug events were the most common reason for treatment discontinuation (13.5%), rather than complications from the intravenous port systems.
With the right training and infrastructure, self-administered therapy empowers patients to manage their treatment safely, reducing the need for prolonged hospital stays while maintaining high standards of care.
doi: 10.1007/s40265-024-02122-4
Changes in microbiota as a function of tuberculosis therapy
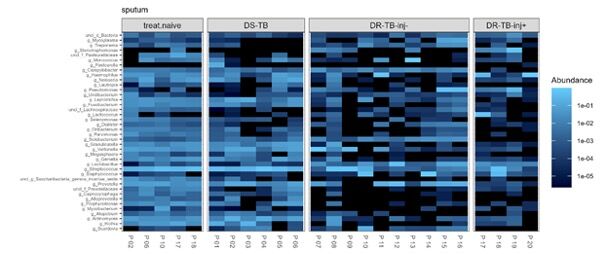
The treatment of patients with drug-resistant tuberculosis is characterized by the number and type of antibiotics and the long duration of therapy, which influence the diversity and composition of the human microbiota. Second-line anti-TB treatment includes several broad-spectrum antibiotics, in particular the injectable aminoglycosides and carbapenems.
In this study, in collaboration with Dr. Mathias Hauptmann from the Cellular Microbiology Research Group, we are investigating how different second-line anti-TB treatment regimens affect the diversity of the human microbiota in the lung and gut in patients with varying degrees of drug-resistant TB.
Standard anti-TB therapy in individuals with susceptible tuberculosis showed only minimal changes in the intestinal and respiratory microbiome within the first 4–6 weeks after initiation of therapy. When intravenous antibiotics from the aminoglycoside and/or carbapenem group were administered during treatment for drug-resistant tuberculosis, this led to a decrease in the diversity of the gut microbiome after 6 weeks. This therapy also decreased the relative diversity of pathobionts in the respiratory tract, potentially leading to an increased risk of worsening tuberculosis and sepsis.
doi: 10.5588/ijtldopen.24.0325
Impact of bedaquiline on treatment outcome in drug-resistant tuberculosis
Background:
Evaluation of novel anti-tuberculosis (TB) drugs for the treatment of multidrug-resistant (MDR)-TB continues to be of high interest on the TB research agenda. We assessed treatment outcomes in patients with pulmonary MDR-TB who received bedaquiline-containing treatment regimens in the Republic of Moldova, a high-burden MDR-TB country.
Methods:
We systematically analysed the SIMETB national electronic TB database and performed a retrospective propensity score-matched comparison of treatment outcomes in a cohort of patients with MDR-TB who started treatment during 2016-2018 with a bedaquiline-containing regimen (bedaquiline cohort) and a cohort of patients treated without bedaquiline (non-bedaquiline cohort).
Results:
Following propensity score matching, 114 patients were assigned to each cohort of MDR-TB patients. Patients in the bedaquiline cohort had higher 6-month sputum culture conversion rate than those in the non-bedaquiline cohort (66.7% versus 40.3%; p<0.001). Patients under bedaquiline-containing regimens had a higher cure rate assessed by both World Health Organization (WHO) and TBnet definitions (55.3% versus 24.6%; p=0.001 and 43.5% versus 19.6%; p=0.004, respectively), as well as a lower mortality rate (8.8% versus 20.2%; p<0.001 and 10.9% versus 25.2%; p=0.01, respectively). In patients who previously failed on MDR-TB treatment, >40% of patients achieved a cure with a bedaquiline-containing regimen.
Conclusions:
Bedaquiline-based MDR-TB treatment regimens result in better disease resolution when compared with bedaquiline-sparing MDR-TB treatment regimens under programmatic conditions in a country with a high burden of MDR-TB.
doi: 10.1183/13993003.02544-2020
Use of TNF antagonists and JAK inhibitors in patients with previous tuberculosis
Tumor necrosis factor (TNF) antagonists and Janus kinase (JAK) inhibitors are important treatment options for chronic inflammatory diseases such as rheumatoid arthritis. There is currently uncertainty about how to manage patients with prior or current tuberculosis who require treatment with TNF antagonists or JAK inhibitors for underlying inflammatory conditions. As part of a systematic review, we searched the medical literature for corresponding reports and identified 368 patients who were treated with TNF antagonists during or after tuberculosis. Only 14 of these patients (3.8 %) had a recurrence of tuberculosis. Of the eleven patients who received a JAK inhibitor during or after tuberculosis, only one patient (9.1 %) had a recurrence of tuberculosis. These low numbers suggest that the use of TNF antagonists and JAK inhibitors may be relatively safe in patients with current or previous TB and the need for further treatment of underlying diseases
doi: 10.1016/j.cmi.2024.04.011
Consensus management recommendations for less common non-tuberculous mycobacterial pulmonary diseases
The 2020 clinical practice guideline for the treatment of non-tuberculous mycobacterial pulmonary disease (NTM-PD) by the American Thoracic Society, European Respiratory Society, European Society of Clinical Microbiology and Infectious Diseases, and Infectious Diseases Society of America; and the 2017 management guideline by the British Thoracic Society covered pulmonary diseases in adults caused by Mycobacterium avium complex, Mycobacterium kansasii, Mycobacterium xenopi, and Mycobacterium abscessus. In order to provide evidence-based recommendations for the treatment of less common non-tuberculous mycobacterial (NTM) species in adult patients without cystic fibrosis or HIV infection, our expert panel group performed systematic literature searches to provide management guidance for pulmonary diseases caused by seven additional organisms: Mycobacterium chelonae, Mycobacterium fortuitum, Mycobacterium genavense, Mycobacterium gordonae, Mycobacterium malmoense, Mycobacterium simiae, and Mycobacterium szulgai. Treatment recommendations were developed by a structured consensus process. The evidence from the scientific literature published in English for treatment recommendations for pulmonary diseases caused by other NTM species was of very low quality, with the exception of M malmoense, and based on the evaluation of case reports and case series. For M malmoense, results from two randomised controlled trials and three retrospective cohort studies provided a better evidence base for treatment recommendations, although the evidence was still of low quality.
doi: 10.1016/S1473-3099(21)00586-7

DZIF – Germany Center for Infection Research
- Clin TB
- Sponsor: DZIF
- Project leader in the research group: Prof. C. Lange
- Project partners: DZIF
- Duration: 2026-2030
- German TB Cohort / TB-samples Bio-Repository
- Sponsor: DZIF
- Project leader in the research group: Dr. Maja Reinmann
- Project partners:
PD Dr. med. A. Rachow (Munich)
Dr. med. Florian Marx (Heidelberg)
Prof. Dr. rer. nat. Christof Geldmacher (Munich)
Dr. rer. nat. Kathrin Held (Munich)
Dr. rer. nat. Olga Baranov (Munich)
Prof. Dr. Dr. Jan Rybniker (Cologne)
Prof. Dr. med. Susanne Herold (Gießen) - Duration: 2026-2030
BMZ (Feredal Ministery for Economic Cooperation and Development) and ekfs (Else Kröner-Fresenius-Stiftung)
- BREATHE
- Sponsor: BMZ and ekfs
- Project leader in the research group: PD Dr. med Thomas Theo Brehm
- Project partners: PV.N. Karazin Kharkiv National University in Kharkiw, Ukraine
- Duration: 2025-2027
- Funding amount: 200.000 Euro
EU-Projects
- UNITE4TB
- Sponsor: EU-IMI
- Project leader in the research group: Prof. C. Lange
- Project partner: 27 international project partners
- Duration: 2021-2028
S-H Society for the Prevention & Control of Tuberculosis & Lung Diseases e.V.
- Access TDMetrics
- Sponsor: S-H Society for the Prevention & Control of Tuberculosis & Lung Diseases e.V.
- Project leader in the research group: Niklas Köhler
- Project partner: University of Hamburg, Institute for Clinical Pharmacy Uppsala University
- Duration: 2023-2026
- Funding amount: 70.000 Euro
- Epigenetic & genetic evaluation of treatment outcome in patients with multidrug-resistent and drug-sensitive tuberculosis
- Sponsor: S-H Society for the Prevention & Control of Tuberculosis & Lung Diseases e.V.
- Project leader in the research group Dr. Maja Reimann
- Project partners:
Prof. Dr. Markus Weckmann (UKSH Lübeck)
Prof. Dr. Folke Brinkmann (UKSH Lübeck) - Duration: 2023-2026
- Validation of a new biomarker (TB22) for the diagnosis and therapy monitoring of tuberculosis
- Sponsor: S-H Society for the Prevention & Control of Tuberculosis & Lung Diseases e.V.
- Project leader in the research group: Dr. Maja Reimann
- Project partners:
- Duration: 2023-2026
- Funding amount: 60.000 Euro
- Impact of sputum-based and sequence-guided individualized therapy on treatment outcomes in drug-resistant TB (T3 study)
- Sponsor: S-H Society for the Prevention & Control of Tuberculosis & Lung Diseases e.V.
- Projec leader in the research group: Prof. Dr. Christoph Lange
- Project partner: Prof. Dr. Keertan Dheda (Cape Town, South Africa)
- Duratation: 2023-2026
Lower Saxony Association for the Fight against Tuberculosis, Lung and Bronchial Diseases e.V.
- detecTB
- Project leader in the research group: PD Dr. med. Thomas Theo Brehm and Dr. med. Niklas Köhler
- Project partner: University Medical Center Hamburg-Eppendorf (UKE)
- Duration: 2025-2027
- Funding amount: 30.000 Euro
Clinical Trial Management
- Coordination of international clinical trials for the development of new TB drugs and new treatment regimens
- Coordination of international studies for the development and validation of new methods for tuberculosis diagnostics and therapy monitoring
- Database management
- Epidemiology and biostatistics
Pulmonology Expertise for Clinical Research
- Pulmonary function testing
- Exercise testing
- Radiological diagnostics
Bronchoscopic Infectious Diagnostics
- Bronchoalveolar lavage (BAL) for the collection of cell suspensions

Cell biological methods
- Preparation of BAL for cytospins and BAL fluids (Immune Cell Analytics)
- Preparation of peripheral mononuclear cells (PBMC)
- Elutriation for the preparation of monocytes (Immune Cell Analytics)
- In vitro infection of BAL cell suspensions with M. tuberculosis (Immune Cell Analytics, Microbial Inflammation Research)
- FACS-analysis for the detection of intracellular cytokines (Immune Cell Analytics)
- ELISPOT for the detection of IFN-g producing T-cells (Immune Cell Analytics)
- ELISA for the quantification of cytokines
- Multiplex-Analysis for the detection of max. 27 cytokines
- Quantitative RT-PCR for the detection of cytokine expression in BAL or PBMNC
- Immunohistochemistry for the intracellular detection of CXCL4 (PF4) (Pathology)
Molecular Biological Methods
- Quantitative RT-PCR for gene detection, e.g. cytokine expression in BAL or PBMCs and mycobacterial DNA
- CRISPR-based PCR technology for detection of M. tuberculosis–specific DNA from blood
- Transcriptome analyses from peripheral blood using RNA sequencing and microarray technology
- Molecular bacterial load assay (MBLA) for detection of M. tuberculosis–specific RNA from sputum, blood, effusions, and tissues
Therapeutic Drug Monitoring
- HPLC–MS²–based quantification of amikacin, amoxicillin, bedaquiline, capreomycin, clavulanic acid, clofazimine, cycloserine, delamanid, ethambutol, isoniazid, kanamycin, levofloxacin, linezolid, meropenem, moxifloxacin, PAS, prothionamide, pyrazinamide, rifabutin, rifampicin, streptomycin, and terizidone from human plasma
- Modeling to optimize drug concentrations in clinical practice
Public relations and consulting activities
- Daily consultative clinical advisory service (TBinfo +49 4537 1880)
- Weekly TBinfo Board
- Course Clinical Tuberculosis
- European Advanced Course for Clinical Tuberculosis
- TBnet Academy
- DanGerouS Mycobacteria Club (Danish–German–Swedish Consortium)

Awards
2026
Oskar Medicine Price 2025: Christoph Lange
2025
Habilitation Award of the Republic of Moldova: Dumitru Chesov
Michael E. DeBakey, M.D. Excellence in Research Award 2025: Anna Mandalakas
Pulmonology: Best Case Report: Johanna Eggeling
PhD Award of the "Kreis Segeberg": Niklas Köhler
DZIF Poster Award 2025: Dariusz Wölk
2024
Fellow of the Royal College of Physicians (FRCP): Christoph Lange
2023
Mentor Award: Anna Maria Mandalakas

2022
2026
Ahmad, R, Ramos, JP, Duarte, R, Lange, C & Van Rest, J 2026, 'Enhancing tuberculosis care in lower and middle-income countries through digital adherence technologies', Breathe, Jg. 22, Nr. 1, S. 250173. https://doi.org/10.1183/20734735.0173-2025
Behrens, E, Köhler, N, Münchow, M, Zielinski, N, Pfaffendorf, C, Grobbel, H-P, Schaub, D, Reimann, M, Sánchez Carballo, PM, Kalsdorf, B, Hillemann, D, Kuhns, M, Hofmann-Thiel, S, Hoffmann, H, Decosterd, LA, Choong, E, Aarnoutse, R, Lange, C & Wicha, SG 2026, 'Towards model-informed precision dosing of clofazimine, moxifloxacin, and terizidone/cycloserine in the treatment of drug-resistant tuberculosis: An external model evaluation study', Tuberculosis, Jg. 157, S. 102744. https://doi.org/10.1016/j.tube.2026.102744
de Rioja, VL, Goovaerts, O, Vidal, M, Amuasi, J, Awuah, AA-A, Mwan-Za-K'a, CK, Mbala-Kingebeni, P, Kibambe, RN, Tshitamba, M, Kazadi, C, Yeshaneh, WE, Hunde, DB, Asres, M, Tajebe, F, Massinga, MM, Maphossa, V, Strauss, R, Ascofare, OM, Monnot, F, Lassout, NIZ, Musa, A, ANTICOV-IMMUNO Consortium, Adriaensen, W & Moncunill, G 2026, 'Determinants of long-term SARS-CoV-2 immune responses in asymptomatic-to-moderate COVID-19 patients in sub-Saharan Africa', BMC Medicine, Jg. 24, Nr. 1, S. 44. https://doi.org/10.1186/s12916-025-04607-9
Kirakosyan, O, Verbree, H, van der Zalm, MM, Nightingale, R, Muñoz-Torrico, M, Rossato Silva, D, Singh, SJ, Centis, R, Duarte, R, Akkerman, OW & Migliori, GB 2026, 'Rehabilitation for individuals with post-tuberculosis lung disease', Breathe, Jg. 22, Nr. 1, S. 250056. https://doi.org/10.1183/20734735.0056-2025
Klingmüller, A, Zuber, S, Hoffmann, AM, Köhler, N, Suárez, I, Preßel, T, Niemann, S, Dreyer, V, Decosterd, LA, Choong, E, Schütz, B, Friesen, I & Rybniker, J 2026, 'Therapeutic drug monitoring-guided treatment of XDR TB with an RpoB I491F mutation-a case report', JAC-antimicrobial resistance, Jg. 8, Nr. 1, S. dlaf251. https://doi.org/10.1093/jacamr/dlaf251
Ma'aruf, SY, Sharma, A, Fox, S, Duarte, R, Lange, C & Tiberi, S 2026, 'Management of adverse events in TB care and active TB drug safety monitoring', Breathe, Jg. 22, Nr. 1, S. 250203. https://doi.org/10.1183/20734735.0203-2025
Moreira-Sousa, D, Martins, B, Aguiar, A, Pinheiro, M, Akkerman, OW, Aksamit, TR, Aliberti, S, Andrejak, C, Daley, CL, van Ingen, J, Lange, C, Lipman, M, Loebinger, MR, Jankovic Makek, M, Morimoto, K, Thomson, R, Wagner, D, Winthrop, KL, Yim, J-J & Duarte, R 2026, 'Management of nontuberculous mycobacterial Pulmonary disease refractory to guideline-based therapy: A systematic review', Pulmonology, Jg. 32, Nr. 1, S. 2632467. https://doi.org/10.1080/25310429.2026.2632467
Tulu, B, Brehm, TT, van Crevel, R, Dallenga, T, DiNardo, AR, Dheda, K, Eggeling, J, Enbiale, W, Gröschel, MI, Hao, J, Kumar, V, van Laarhoven, A, Londt, R, Prosser, G, Randall, P, Reiling, N, Rybniker, J, Schaible, UE, Schurr, E, Suarez, I, Theobald, SJ, Wilkinson, RJ & Lange, C 2026, 'Host- and pathogen-related determinants of pulmonary versus extrapulmonary tuberculosis', European Respiratory Review, Jg. 35, Nr. 179. https://doi.org/10.1183/16000617.0174-2025
2025
Adu Gyamfi, CG, Seeger, A, Mulengwa, D, Vasiliu, A, Carratala-Castro, L, Mtafya, B, Maphalala, N, Munguambe, S, Saavedra, B, Ness, T, Maphalala, G, Acacio, S, Mambuque, E, Ehrlich, J, Mejia, R, Ziyane, M, Kirchner, HL, Lange, C, Kay, A, Garcia-Basteiro, AL, Mandalakas, A, DiNardo, AR & Stool4TB Global Partnership 2025, 'Stool-Based Molecular Tuberculosis Treatment Monitoring: A Faster Means for Detecting Persistent Mycobacteria Compared to Phenotypic Culture', Open forum infectious diseases, Jg. 12, Nr. 8, S. ofaf345. https://doi.org/10.1093/ofid/ofaf345
Belheouane, M, Kalsdorf, B, Niemann, S, Gaede, KI, Lange, C, Heyckendorf, J & Merker, M 2025, 'Serratia sp. traits distinguish the lung microbiome of patients with tuberculosis and non-tuberculous mycobacterial lung diseases', PLOS ONE, Jg. 20, Nr. 6, S. e0325362. https://doi.org/10.1371/journal.pone.0325362
Boffa, J, Vambe, D, Khosa, C, José, B, Ndjeka, N, Nkomo, T, Kay, AW, Mandalakas, AM, Mvusi, L, Omar, SV, Thi, S, Velen, K, Charalambous, S & Rangaka, MX 2025, 'TB elimination in Southern Africa: overview and critical reflection', IJTLD open, Jg. 2, Nr. 7, S. 381-387. https://doi.org/10.5588/ijtldopen.25.0050
Brehm, TT, Akkerman, OW, Sotgiu, G, Tiberi, S, Chang, K-C, Dheda, K, Duarte, R, Vambe, D, Udwadia, ZF, Chesov, D, Mendelson, M, Iswari Saktiawati, AM, van Ingen, J, Eyuboglu, FO, Tängdén, T, Quang Vo, LN, Riccardi, N, Moschos, C, Friedland, JS, Lillebaek, T, Chandy, SJ, Caminero, JA, Thwaites, G, Gandra, S, Thursky, K, George, IA, Konstantynovska, O, Fatima, R, Yim, J-J, Kwak, N, Olaru, ID, Gillespie, SH, Kherabi, Y, Perl, SH, Grønningen, E, Rodrigues, C, Bjerrum, S, Bange F, Cox V, Cirillo DM, Saluzzo F, Hara GL, Wagner D, Ismail N, Sloan DJ, Eshun-Wilsonova I, Zeng M, Cantero C, Vasankari T, Mandalakas AM, Kay A, Ness T, Torrico MM, Günther G, Kuksa L, Guglielmetti L, García-Basteiro AL, Marks GB, Pulcini C & Lange C 2025, 'Clinical standards for antimicrobial stewardship in TB care', IJTLD open, Jg. 2, Nr. 12, S. 716-726. https://doi.org/10.5588/ijtldopen.25.0522
Brehm, TT, Köhler, N, Grobbel, H-P, Welling, J, Mandalakas, AM, Fava, V, Schurr, E & Lange, C 2025, 'High risk of drug-resistant tuberculosis in IGRA-negative contacts: should preventive treatment be considered?', Infection. https://doi.org/10.1007/s15010-024-02470-z
Budde, K, Lange, C, Reimann, M, Zielinski, N, Meiwes, L, Köhler, N, Sanchez Carballo, P & DZIF-TB cohort study group 2025, 'A novel method for detecting Lipoarabinomannan in urine with the promise of meeting the WHO target product profile for the diagnosis of tuberculosis', Tuberculosis, Jg. 152, S. 102619. https://doi.org/10.1016/j.tube.2025.102619
Carratalà Castro, L, Munguambe, S, Kay, A, Ssengooba, W, Mulengwa, D, Acácio, S, Ehrlich, J, DiNardo, AR, Vasiliu, A, Seeger, A, Mambuque, E, Adu-Gyamfi, CG, Timoteo, H, Lange, C, Hermans, S, Mandalakas, A, López-Varela, E & García-Basteiro, AL 2025, 'Estimating the Accuracy and Additionality of TB-LAM to Diagnose TB in Children Without HIV (AccuLAM)', CLINICAL INFECTIOUS DISEASES . https://doi.org/10.1093/cid/ciaf525
Carrero Longlax, S, Koster, KJ, Kamat, AM, Lozano, M, Lerner, SP, Hannigan, R, Nishiguchi, T, Abhimanyu, Sheikh, D, Ladki, M, Portillo, A, Koirala, A, Patel, TD, Spieler, Z, Benjamin, AB, Lebedev, M, Ofili, TU, Hutchison, RW, Udeani, G, Opperman, LA, Neal, G, Mandalakas, AM, Netea, MG, Arditi, M, Avalos, P, Grimm, SL, Coarfa, C, Cirillo, JD & DiNardo, AR 2025, 'BCG-Induced DNA Methylation Changes Improve Coronavirus Disease 2019 Vaccine Immunity Without Decreasing the Risk for Severe Acute Respiratory Syndrome Coronavirus 2 Infection', Open forum infectious diseases, Jg. 12, Nr. 1, S. ofaf007. https://doi.org/10.1093/ofid/ofaf007
Chesov, D, Reimann, M, Mukherjee, T, Tewatia, K, Konstantynovska, O, David, A, Rusu, D, Ciobanu, N, Crudu, V & Lange, C 2025, 'High rates of acquired resistance to fluoroquinolones, bedaquiline and linezolid in patients failing treatment against drug-resistant tuberculosis in the Republic of Moldova', CLINICAL MICROBIOLOGY AND INFECTION . https://doi.org/10.1016/j.cmi.2025.09.003
Crudu, V, Chesov, D, Codreanu, A, Turcanu, N, Ciobanu, N, Nepoliuc, L & Rusu, D 2025, 'Diagnostic accuracy study of STANDARD TB-Feron FIA and STANDARD TB-Feron ELISA tests for tuberculosis infection diagnosis in Eastern European setting', Clinical Tuberculosis and Other Mycobacterial Diseases, Jg. 39, S. 100518. https://doi.org/10.1016/j.jctube.2025.100518
Cukoski, S, Brehm, TT, Büttner, S, Van Praet, J, Dolff, S, Eberwein, L, Falces-Romero, I, Cornely, OA, Wanken, M, Müller, R-U, Burst, V & Koehler, FC 2025, 'Design and set-up of the leptospirosis registry LeptoScope for epidemiology, outbreaks and clinical studies on human leptospirosis', Frontiers in public health, Jg. 13, S. 1687249. https://doi.org/10.3389/fpubh.2025.1687249
D'Silva, OA, Lancione, S, Ananthakrishnan, O, Addae, A, Shrestha, S, Alsdurf, H, Thavorn, K, Mzizi, N, Vasilu, A, Kay, A, Mandalakas, AM & Zwerling, AA 2025, 'The catastrophic cost of TB care: Understanding costs incurred by individuals undergoing TB care in low-, middle-, and high-income settings - A systematic review', PLOS global public health, Jg. 5, Nr. 4, S. e0004283. https://doi.org/10.1371/journal.pgph.0004283
DiNardo, AR, Sabiiti, W, Gillespie, SH, Georghiou, SB, Heinrich, N, Hittel, N, Taghlabi, S, Carrero Longlax, D, Kohli, M, Panzner, U, Musia, C, Lange, C, Vasiliu, A, Arts, RJW, Mandalakas, AM, Ruhwald, M, Stuyver, LJ & van Crevel, R 2025, 'Inclusion of patient-centered, non-microbiological endpoints and biomarkers in tuberculosis drug trials', Frontiers in antibiotics, Jg. 4, S. 1570989. https://doi.org/10.3389/frabi.2025.1570989
Dudnyk, A, Lutchmun, W, Duarte, R, Lange, C & Svensson, EM 2025, 'The importance of getting the dose right in the treatment of tuberculosis', Breathe, Jg. 21, Nr. 1, S. 240177. https://doi.org/10.1183/20734735.0177-2024
Epple, H-J, Domaszewska, T, Brünneck, A-CV, Furth, C, Dommerich, S, Lange, C, Schöning, DV, Maschmeyer, G, Bös, L, Schwartz, S, Schneider, T & Mathas, S 2025, 'At the crossroads of infection and malignancy: the challenge of tuberculosis in migrating populations - Case Report and Epidemiologic Analysis', BMC INFECTIOUS DISEASES . https://doi.org/10.1186/s12879-025-12043-6
Essandoh, MN, Mackroth, MS, Brehm, TT, Michelitsch, P, Mbassi, FE, Rakotonirinalaloa, M, Ijagbemi, K & Ramharter, M 2025, 'Malaria risk perceptions and barriers for effective prophylaxis among sub-Saharan African `visiting friends and relatives´ travellers in Hamburg, Germany', Travel medicine and infectious disease, S. 102858. https://doi.org/10.1016/j.tmaid.2025.102858
Feischen, M, Jordan, S, Kalsdorf, B & Schmiedel, S 2025, 'Komplikativer Verlauf einer Infektion mit Mycobacterium marinum – „Nihil nocere“ bei Differenzialdiagnostik und Therapie', Deutsche medizinische Wochenschrift (1946), Jg. 150, Nr. 17, S. 1046-1049. https://doi.org/10.1055/a-2615-9925
Frings, VG, Lâm, T-T, Lange, C, Goebeler, M & Stoevesandt, J 2025, 'Disseminated abscesses due to Mycobacterium wolinskyi and Mycobacterium mageritense: An unusual mixed infection', JAAD case reports, Jg. 61, S. 62-64. https://doi.org/10.1016/j.jdcr.2025.04.024
Garcia-Prats, AJ, Garcia-Cremades, M, Cox, V, Kredo, T, Dunbar, R, Schaaf, HS, Seddon, JA, Furin, J, Achar, J, Radke, K, Sachs, T, Abubakirov, A, Ahmed, S, Akkerman, OW, Al Ani, NA, Amanullah, F, Ahmad, N, Anderson, LF, Asfaw, M, Bango, F, Bauer, T, Becerra, M, Boeree, M, Brinkmann, F, Brown, R, Brust, J, Campbell, JR, Carvalho, AC, Carvalho, I, Cegielski, JP, Centis, R, Chan, ED, Chauhan, S, Chiang, SS, Chan, P-C, D'Ambrosio, L, Dalcolmo, M, Daneilyan, N, de Vries, G, Draper, HR, endTB Study Group, Fairlie, L, Francis, JR, Franke, M, Gegia, M, Restrepo, CG, Guenther, A, Gureva, T, Haecker, B, Harausz, E, Hewison, C, Hicks, RM, Huerga, H, Hughes, J, Isaakidis, P, Syed M, K, Khan, MA, Kotrikadze, T, Kuksa, L, Lachenal, N, Lange, C, Lecca, L, Lopez-Varela, E, Lucena, S, Mariandyshev, A, Mattoo, S, Mendez-Echevarria, A, Migliori, GB, Mitnick, CD, Mohr-Holland, E, Mulanda, W, Murzabakova, T, Myrzalieve, B, Ndjeka, N, Niemann, S, Ozere, I, Padayatchi, N, Parmar, M, Parpieva, N, Manzur-Ul-Alam, M, Rybak, N, Sachdeva, KS, Salmon, K, Santiago-Garcia, B, Schaub, D, Shah, I, Shah, NS, Shah, V, Sharma, S, Shim, TS, Shin, SS, Sinha, A, Skrahina, A, Solanki, H, Solans, BP, Soriano-Arandes, A, Toktogonova, A, van der Werf , TS, Velásquez, GE, Williams, B, Yim, J-J, Savic, R & Hesseling, AC 2025, 'Characteristics of children and adolescents with multidrug-resistant and rifampicin-resistant tuberculosis and their association with treatment outcomes: a systematic review and individual participant data meta-analysis', Lancet child & adolescent health, Jg. 9, Nr. 2, S. 100-111. https://doi.org/10.1016/S2352-4642(24)00330-4
Goletti, D, Cirillo, DM, Lange, C, Tiberi, S, Günther, G, Petrone, L, Kuksa, L, Opota, O, Akkerman, O, Podlekareva, D, Guglielmetti, L & Duarte, R 2025, 'The Empty Medicine Cabinet: Urgent Action Needed to Resolve TB Drug Shortages in Europe', INTERNATIONAL JOURNAL OF INFECTIOUS DISEASES , S. 108022. https://doi.org/10.1016/j.ijid.2025.108022
Guglielmetti, L, Khan, U, Velásquez, GE, Gouillou, M, Ali, MH, Amjad, S, Kamal, F, Abubakirov, A, Ardizzoni, E, Baudin, E, Bektassov, S, Berry, C, Bonnet, M, Chavan, V, Coutisson, S, Dakenova, Z, de Jong, BC, Dinh, LV, Ferlazzo, G, Kirakosyan, O, Lachenal, N, Lecca, L, McIlleron, H, Mikanda, KK, Mucching-Toscano, S, Mulders, W, Mushtaque, H, Nahid, P, Nguyen, DV, Nguyen, NV, Oyewusi, L, Motta, I, Panda, S, Patil, S, Pham, TH, Phan, DT, Phan, HTT, Phillips, PPJ, Ruiz, J, Rupasinghe, P, Salahuddin, N, Sanchez-Garavito, E, Seung, KJ, Asfaw, MT, Vargas Vasquez, D, Rich, ML, Varaine, F, Mitnick, CD & endTB-Q Clinical Trial Team 2025, 'Bedaquiline, delamanid, linezolid, and clofazimine for rifampicin-resistant and fluoroquinolone-resistant tuberculosis (endTB-Q): an open-label, multicentre, stratified, non-inferiority, randomised, controlled, phase 3 trial', Lancet Respiratory Medicine. https://doi.org/10.1016/S2213-2600(25)00194-8
Hoelscher, M, Barros-Aguirre, D, Dara, M, Heinrich, N, Sun, E, Lange, C, Tiberi, S & Wells, C 2025, 'Corrigendum to "Candidate anti-tuberculosis medicines and regimens under clinical evaluation" [Clin Microbiol Infect 30(9) (2024) 1131-1138]', CLINICAL MICROBIOLOGY AND INFECTION . https://doi.org/10.1016/j.cmi.2025.10.001
Heinrich, F, Wong, TLE, Graf, W, Dost, K, Brennecke, A, Kowalski, V, van Rüth, V, Iwersen-Bergmann, S, Hajek, A, König, H-H, Renné, T, Brehm, TT, Pfefferle, S, Schulze Zur Wiesch, J, Dandri, M, Aepfelbacher, M, Püschel, K, Ondruschka, B, Lütgehetmann, M & Stallbaum, F 2025, 'Prevalence and risk factors of viral hepatitis and HIV among people experiencing homelessness in Germany based on a nationwide study', Scientific Reports, Jg. 15, Nr. 1, S. 32571. https://doi.org/10.1038/s41598-025-18552-3
Idris, R, Urschel, R, Lange, C & Sester, M 2025, 'Evidenzbasierter Einsatz von Interferon-Gamma Release Assays in der Tuberkulosediagnostik', Zeitschrift für Pneumologie. https://doi.org/10.1007/s10405-025-00651-7
Inman, B, Butler, J, George-Nichol, S, Kovalenko, G, Savidge, T, Vergnetti, Y, Pongratz, C, Bee, E, DiNardo, AR, Kay, A, Mandalakas, A, Bortz, E & Ness, TE 2025, 'Application of FreezeTB, a targeted nanopore sequencing assay, for identification of drug resistance and lineages among pulmonary tuberculosis cases in Alaska', Microbiology spectrum, S. e0233525. https://doi.org/10.1128/spectrum.02335-25
Kasule, GW, Hermans, S, Acacio, S, Kay, A, Nsubuga, JK, Fernández-Escobar, C, Shiba, N, Carratalá-Castro, L, Semugenze, D, Mwachan, P, Munguambe, S, Ehrlich, J, Lopez-Varela, E, DiNardo, AR, Cobelens, F, Lange, C, Joloba, M, Mandalakas, AM, Ssengooba, W, García-Basteiro, AL & Stool4TB Global Partnership 2025, 'Performance of stool Xpert MTB/RIF Ultra for detection of Mycobacterium tuberculosis among adults living with HIV: a multicentre, prospective diagnostic study', The Lancet. Microbe, S. 101085. https://doi.org/10.1016/j.lanmic.2025.101085
Kherabi, Y, Skouvig Pedersen, O, Lange, C, Chesov, D, Bénézit, F, Codecasa, LR, Dudnyk, A, Kiria, N, Konstantynovska, O, Marigot-Outtandy, D, Panciu, T-C, Poignon, C, Sasi, S, Schaub, D, Solodovnikova, V, Vasiliauskaitè, L, Yeghiazaryan, L, Günther, G, Guglielmetti, L & TBnet/ESGMYC XDR-TB Study Group 2025, 'Treatment outcomes of extensively drug-resistant tuberculosis in Europe: a retrospective cohort study', The Lancet regional health. Europe, Jg. 56, S. 101380. https://doi.org/10.1016/j.lanepe.2025.101380
Kirakosyan, O, Reimann, M, Andersen, AB, Bjarnason, A, Bakos, Á, Dyrhol-Riise, AM, McLaughlin, AM, Nita, C, Pieridou, D, Chesov, D, Davidavičienė, EV, Günther, G, Atshemyan, H, Muylle, I, Solovic, I, Bruchfeld, J, Manika, K, Kuksa, L, Codecasa, LR, Stosic, M, Skowroński, M, Makek, MJ, Fréchet Jachym, M, Knappik, M, Santin, M, Yatskevich, N, Konstantynovska, O, Akkerman, O, Svetina, P, Viiklepp, P, Duarte, R, Zeynel, S, Togonidze, T, Vasankari, T, Parris, V, Özkara, Ş, Lange, C & Brehm, TT 2025, 'Use of putative hepatoprotective agents as an adjunct to anti-TB treatment in Europe', IJTLD open, Jg. 2, Nr. 2, S. 101-106. https://doi.org/10.5588/ijtldopen.24.0498
Konstantynovska, O, Synenko, T, Honcharenko, A, Volobuieva, O, Liadova, T, Reimann, M, Lange, C & Chesov, D 2025, 'Fluoroquinolone Resistance in Drug-Resistant Tuberculosis, Kharkiv, Ukraine, 2019–2023', EMERGING INFECTIOUS DISEASES, Jg. 31, Nr. 3, 31 (3), S. 615-617. https://doi.org/10.3201/eid3103.241675
Köhler, N, Otto-Knapp, R, Heinrich, N, Lange, C & Brehm, TT 2025, 'Therapie der Tuberkulose bei Erwachsenen', Deutsche medizinische Wochenschrift (1946), Jg. 150, Nr. 20, S. 1207-1215. https://doi.org/10.1055/a-2612-2364
Kuksa, L, Andrejak, C, Haecker, B, Bothamley, G, Calcagno, A, Cirillo, DM, Duarte, R, Fatima, R, Ferlazzo, G, Guglielmetti, L, Günther, G, Hewison, C, Horsburgh, CR, Jäger, T, Kalancha, Y, Otto-Knapp, R, Kranzer, K, Lillebaek, T, Marks, G, Middelkoop, K, Motta, I, Rabinova, V, Sommerfeld, P, Tattevin, P & Lange, C 2025, 'Urgent request for pretomanid label expansion to align with WHO guidelines and improve treatment accessibility and efficacy', IJTLD open, Jg. 2, Nr. 3, S. 117-119. https://doi.org/10.5588/ijtldopen.25.0152
Lange, B, Brehm, TT, Arend, SM, Arias-Guillén, M, Bakker, M, Berastegui, C, Babiker, M, Charif, R, Duarte, R, Flick, H, Hofland, RW, Ismail, J, Kniepeiss, D, Krepel, J, Krishnan, N, Kuijpers, DL, Kunst, H, van Leth, F, Lezaic, V, Los-Arcos, I, Machová, J, Milburn, H, Morais, SA, Kon, OM, Osoro-Suarez, C, Pessegueiro Miranda, H, Pesut, D, Rahman, A, Reischig, T, Sánchez-Montalvá, A, Spohn, HE, Stegenga, MT, de Vries, APJ, Wagner, D, Wobser, R, Lange, C & Sester, M 2025, 'Tuberculosis incidence in solid organ transplant recipients in Europe: A multicenter TBnet cohort study', Journal of infection, Jg. 92, Nr. 1, S. 106668. https://doi.org/10.1016/j.jinf.2025.106668
Lange, C, Barry, C & Sotgiu, G 2025, 'Linezolid for the treatment of drug-resistant tuberculosis', The European respiratory journal, Jg. 66, Nr. 2. https://doi.org/10.1183/13993003.00927-2025
Lange, C, Bothamley, G, Günther, G, Guglielmetti, L, Kontsevaya, I, Kuksa, L, Lange, B, Lorent, N, Saluzzo, F, Sester, M, Tebruegge, M, Tunesi, S, Tweed, C & Tuberculosis Network European Trials group (TBnet) 2025, 'A Year in Review on Tuberculosis and Non-tuberculous Mycobacteria Disease: A 2025 Update for Clinicians and Scientists', Pathogens and Immunity, Jg. 10, Nr. 2, S. 1-45. https://doi.org/10.20411/pai.v10i2.791
Lange, C, Schaberg, T, Otto-Knapp, R & für die Autorinnen und Autoren der Tuberkulose-Leitliniengruppe für Deutschland, Österreich und die Schweiz: 2025, 'Update: Therapie der medikamentenresistenten Tuberkulose (DR-TB) in Deutschland, Österreich und der Schweiz', Pneumologie (Stuttgart, Germany). https://doi.org/10.1055/a-2731-0729
Leo, F, Polsfuss, S, Przewosnik, A-S, Grohé, C & Lange, C 2025, 'Mycobacterium florentinum pulmonary disease: a case report and review of the literature', BMC INFECTIOUS DISEASES . https://doi.org/10.1186/s12879-025-12321-3
Lima, AV, Acácio, S, Cossa, H, Hermans, S, Kay, A, Ssengooba, W, Mandalakas, A, Lange, C, Garcia-Basteiro, A, Munguambe, K & Stool4 TB Global Partnership 2025, 'Perceived usability, acceptability, and feasibility of stool-based qPCR TB diagnostics: perspectives from healthcare providers in Manhiça District, southern Mozambique', BMC INFECTIOUS DISEASES , Jg. 25, Nr. 1, S. 757. https://doi.org/10.1186/s12879-025-11117-9
Lima, AV, Cossa, H, Djive, H, Cossa, O, Cumbe, M, Acácio, S, Nkala, B, Nsubuga Kikoyo, J, Carratala-Castro, L, Ehrlich, J, Hermans, S, Kay, A, Ssengooba, W, Mandalakas, A, Lange, C, Enguita-Fernàndez, C, Munguambe, K, Garcia-Basteiro, AL & Stool4TB Global Partnership 2025, 'Gaps in TB-related knowledge and practices: An assessment of health care seeking behavior among adults with HIV and caregivers of paediatric patients with presumptive TB symptoms in Manhiça district, southern Mozambique', PLOS global public health, Jg. 5, Nr. 8, S. e0004734. https://doi.org/10.1371/journal.pgph.0004734
Madison, M, Vambe, D, Thi, SS, Ziyane, M, Shiba, N, Nkala, BB, Ness, T, Lima, AV, Dlamini, S, Ngwenya, S, Mandalakas, A & Kay, A 2025, 'Healthcare providers' knowledge, attitudes, and perceptions from using targeted sequencing to diagnose and manage drug-resistant tuberculosis (DR-TB) in Eswatini', PLOS global public health, Jg. 5, Nr. 6, S. e0004718. https://doi.org/10.1371/journal.pgph.0004718
Mandalakas, AM, Casenghi, M & Zwerling, A 2025, 'Childhood tuberculosis: robust funding and integrated solutions needed now', Lancet child & adolescent health. https://doi.org/10.1016/S2352-4642(25)00245-7
Mischnik, A, Kuhns, M, Meiwes, L, Pichlo, S, Gaudlitz, J, Zielinski, N & Brehm, TT 2025, 'Neue Methoden der Tuberkulose-Diagnostik', Deutsche medizinische Wochenschrift (1946), Jg. 150, Nr. 20, S. 1198-1206. https://doi.org/10.1055/a-2612-2417
Moreira-Sousa, D, Martins, B, Aguiar, A, Pinheiro, M, Akkerman, O, Aksamit, TR, Aliberti, S, Andrejak, C, Daley, CL, van Ingen, J, Lange, C, Lipman, M, Loebinger, MR, Jankovic Makek, M, Morimoto, K, Thomson, RM, Wagner, D, Winthrop, KL, Yim, J-J & Duarte, R 2025, 'Consensus on Management of Refractory Nontuberculous Mycobacterial Pulmonary Disease', The European respiratory journal. https://doi.org/10.1183/13993003.00400-2025
Ness, TE, Ziyane, M, Maphalala, N, Seeger, A, Vasiliu, A, Khumalo, W, Thunzini, M, Dlamini, S, Maphalala, G, Gascua, C, Lange, C, Meyer, S, Inman, B, Dreyer, V, Utpatel, C, Niemann, T, DiNardo, A, Kay, A, Niemann, S & Mandalakas, A 2025, 'Screening and targeted sequencing of stool for microbiologic confirmation and drug resistance determination in paucibacillary tuberculosis', PLOS global public health, Jg. 5, Nr. 11, S. e0005243. https://doi.org/10.1371/journal.pgph.0005243
Nkala, BB, Kay, A, Vasiliu, A, Mandalakas, A, Nkomo, T, Mdluli-Dlamini, L, Ngwenya, S, Thi, SS, Masina, S, Sibanda, J, Tsela, S & Vambe, D 2025, 'An assessment of video-observed treatment as an adherence support tool for patients with drug-resistant TB', IJTLD open, Jg. 2, Nr. 12, S. 763-768. https://doi.org/10.5588/ijtldopen.25.0370
Nkomo, T, Udwadia, Z, Vambe, D, van Rie, A, Thi, SS, Stillo, J, Stambekova, A, Sinha, A, Rich, ML, Reuter, A, Patel, J, Otto-Knapp, R, Motta, I, Mesic, A, McKenna, L, Maru, S, Lessem, E, Lange, C, Kiria, N, Kherabi, Y, Günther, G, Guglielmetti, L, Decroo, T, Chen, L, Ashesh, A, Abubakirov, A & Furin, J 2025, 'Clinical best practices for caring for people with expanded resistance to newer TB drugs', IJTLD open, Jg. 2, Nr. 6, S. 315-323. https://doi.org/10.5588/ijtldopen.25.0240
Peterka, M, Krieger, D, Khatamzas, E, Denkinger, C, Köhler, N, Lange, C, Macholz, M, Lübbert, C, Daller, S, Knappik, M, Kuhns, M, Häcker, B, Polsfuß, S, Bauer, T & Otto-Knapp, R 2025, 'Safety of high dose bedaquiline treatment in extensively drug-resistant (XDR)-TB patients', The European respiratory journal. https://doi.org/10.1183/13993003.01496-2025
Pichlo, S, Carballo, PS, Reimann, M, Zielinski, N, Shuaib, YA, Köhler, N, Andres, S, Heyckendorf, J, Kalsdorf, B, Olaru, ID, Salzer, HJF & Lange, C 2025, 'Lipoarabinomannan (LAM) for the diagnosis of tuberculosis from sputum', Journal of infection, Jg. 91, Nr. 6, S. 106662. https://doi.org/10.1016/j.jinf.2025.106662
Pinheiro, M, Aguiar, A, Moreira, DN, Akkerman, OW, Al-Suwaidi, Z, Alffenaar, J-WC, Arandjelović, I, Brito, U, de Colombani, P, Curcic, R, Garcia-Basteiro, AL, Goletti, D, Günther, G, Ibraim, E, Kapata, N, Lange, C, Lipman, M, Jankovic Makek, M, Marais, BJ, Mariandyshev, A, Magis-Escurra, C, Migliori, GB, Sánchez Montalvá, A, Nanovic, Z, Palmero, DJ, Priwitzer, M, Raviglione, MCB, Silva, DR, Salzer, HJF, Schwarzbach, C, Spruijt, I, Winthrop, KL, Udwadia, Z, Vasankari, T, Vilaplana, C & Duarte, R 2025, 'A multinational Delphi consensus on tuberculosis screening of migrants in Europe', ERJ Open Research, Jg. 11, Nr. 6. https://doi.org/10.1183/23120541.00574-2025
Reimann, M, Avsar, K, DiNardo, AR, Goldmann, T, Günther, G, Hoelscher, M, Ibraim, E, Kalsdorf, B, Kaufmann, SHE, Köhler, N, Mandalakas, AM, Maurer, FP, Müller, M, Nitschkowski, D, Olaru, ID, Popa, C, Rachow, A, Rolling, T, Salzer, HJF, Sanchez-Carballo, P, Schuhmann, M, Schaub, D, Spinu, V, Terhalle, E, Unnewehr, M, Zielinski, NJ, Heyckendorf, J & Lange, C 2025, 'The TB27 Transcriptomic Model for Predicting Mycobacterium tuberculosis Culture Conversion', Pathogens and Immunity, Jg. 10, Nr. 1, S. 120-139. https://doi.org/10.20411/pai.v10i1.770
Sester, M, Altet-Gomez, N, Andersen, ÅB, Arias-Guillén, M, Avsar, K, Bakken Kran, A-M, Bothamley, G, Nordholm Breschel, AC, Brown, J, Chesov, D, Ciobanu, N, Cirillo, DM, Crudu, V, de Souza Galvao, M, Dilektasli, AG, Dominguez, J, Duarte, R, Dyrhol-Riise, AM, Goletti, D, Hoffmann, H, Ibraim, E, Kalsdorf, B, Krawczyk, M, Kunst, H, Lange, B, Lipman, M, Matteelli, A, Milkiewicz, P, Neyer, D, Nitschke, M, Oral, HB, Palacios-Gutiérrez, JJ, Petruccioli, E, Raszeja-Wyszomirska, J, Ravn, P, Rupp, J, Spohn, H-E, Toader, C, Villar-Hernandez, R, Wagner, D, van Leth, F, Martinez, L, Pedersen, OS & Lange, C 2025, 'Correction to Diagnostic accuracy and predictive value of the QuantiFERON-TB gold plus assay for tuberculosis in immunocompromised individuals: a prospective TBnet study Lancet Reg Health Eur 57 (2025) 101416 LLRHEUROPE-D-25-00310', The Lancet regional health. Europe, Jg. 59, S. 101523. https://doi.org/10.1016/j.lanepe.2025.101523
Sester, M, Altet-Gomez, N, Andersen, ÅB, Arias-Guillén, M, Avsar, K, Bakken Kran, A-M, Bothamley, G, Nordholm Breschel, AC, Brown, J, Chesov, D, Ciobanu, N, Cirillo, DM, Crudu, V, de Souza Galvao, M, Dilektasli, AG, Dominguez, J, Duarte, R, Dyrhol-Riise, AM, Goletti, D, Hoffmann, H, Ibraim, E, Kalsdorf, B, Krawczyk, M, Kunst, H, Lange, B, Lipman, M, Matteelli, A, Milkiewicz, P, Neyer, D, Nitschke, M, Oral, HB, Palacios-Gutiérrez, JJ, Petruccioli, E, Raszeja-Wyszomirska, J, Ravn, P, Rupp, J, Spohn, H-E, Toader, C, Villar-Hernandez, R, Wagner, D, van Leth, F, Martinez, L, Pedersen, OS & Lange, C 2025, 'Diagnostic accuracy and predictive value of the QuantiFERON-TB gold plus assay for tuberculosis in immunocompromised individuals: a prospective TBnet study', The Lancet regional health. Europe, Jg. 57, S. 101416. https://doi.org/10.1016/j.lanepe.2025.101416
Tchakounte Youngui, B, Tchounga, BK, Atwine, D, Vasiliu, A, Cuer, B, Simo, L, Okello, R, Tchendjou, P, Kuate Kuate, A, Turyahabwe, S, Cohn, J, Graham, SM, Casenghi, M & Bonnet, M 2025, 'Safety of 3-month rifampicin-isoniazid TPT in child household contacts in a community-based intervention', INTERNATIONAL JOURNAL OF TUBERCULOSIS AND LUNG DISEASE, Jg. 29, Nr. 2, S. 67-74. https://doi.org/10.5588/ijtld.24.0311
Vasiliu, A, Carratala-Castro, L, Seeger, A, Ehrlich, J, Nkala, B, Ness, T, Cumbe, MM, Mulengwa, D, Munguambe, S, Mtafya, B, Mambuque, E, Shiba, N, Acacio, S, Komba, L, Adu-Gyamfi, CG, Kirchner, HL, Lange, C, DiNardo, AR, Garcia-Basteiro, A, Mandalakas, AM, Kay, A & Stool4TB Global Partnership 2025, 'Performance of a novel stool quantitative polymerase chain reaction assay for pediatric tuberculosis detection in sub-Saharan Africa', Journal of the Pediatric Infectious Diseases Society. https://doi.org/10.1093/jpids/piaf045
Vasiliu, A, Cristea, V, Stoycheva, K, Rosales-Klintz, S, Lange, C, Zenner, D & Ködmön, C 2025, 'Shifting tuberculosis dynamics in the EU/EEA: geographical and drug resistance trends among people of foreign origin, 2019 to 2023', Eurosurveillance, Jg. 30, Nr. 11. https://doi.org/10.2807/1560-7917.ES.2025.30.11.2500173
Vonasek, BJ, Marcy, O, Armour, J, Casenghi, M, Cazes, C, Chisti, MJ, d'Elbée, M, Huerga, H, Hewison, C, Lancioni, CL, Lungu, PS, McCollum, ED, Musiime, V, Mvalo, T, Seddon, JA, Steenhoff, AP, Thomas, TA, Tovar, M, Vasiliu, A, Garcia-Prats, A & Chabala, C 2025, 'Leveraging nutritional rehabilitation and tuberculosis programmes to tackle tuberculosis and severe acute malnutrition in children', Lancet child & adolescent health. https://doi.org/10.1016/S2352-4642(25)00062-8
Woelk, D, Meiwes, L, Ciobanu, N, Crudu, V, Comanac, A-M, Kulcitkaia, S, Vasiliu, A, Mandalakas, AM, Lange, C, Brehm, TT & Chesov, D 2025, 'Non-invasive diagnosis of pulmonary tuberculosis using face mask sampling: A prospective study in adults', CLINICAL MICROBIOLOGY AND INFECTION . https://doi.org/10.1016/j.cmi.2025.12.017
Youngquist, BM, Saliba, J, Kim, Y, Cutro, TJ, Lyon, CJ, Olivo, J, Ha, N, Fine, J, Colman, R, Vergara, C, Robinson, J, LaCourse, S, Garfein, RS, Catanzaro, DG, Lange, C, Perez-Then, E, Graviss, EA, Mitchell, CD, Rodwell, T, Ning, B & Hu, TY 2025, 'Rapid tuberculosis diagnosis from respiratory or blood samples by a low cost, portable lab-in-tube assay', Science translational medicine, Jg. 17, Nr. 793, S. eadp6411. https://doi.org/10.1126/scitranslmed.adp6411
Zielinski, N, Brehm, TT, Köhler, N, Sánchez Carballo, P, Schaub, D, Lange, C & Reimann, M 2025, 'Analysis of gene expression in patients prior to TB treatment to identify those associated with pyrazinamide-hepatotoxicity', IJTLD open, Jg. 2, Nr. 8, S. 497-499. https://doi.org/10.5588/ijtldopen.25.0189
2024
Abhimanyu, Longlax, SC, Nishiguchi, T, Ladki, M, Sheikh, D, Martinez, AL, Mace, EM, Grimm, SL, Caldwell, T & Portillo Varela, A et al. 2024, 'TCA metabolism regulates DNA hypermethylation in LPS and Mycobacterium tuberculosis-induced immune tolerance', PROCEEDINGS OF THE NATIONAL ACADEMY OF SCIENCES OF THE UNITED STATES OF AMERICA, Jg. 121, Nr. 41, S. e2404841121. https://doi.org/10.1073/pnas.2404841121
Argel, M, Conde, M, Vieira, M, Lange, C, Magis-Escurra, C & Duarte, R 2024, 'Screening of refugees from Ukraine for TB: a TBnet survey', INTERNATIONAL JOURNAL OF TUBERCULOSIS AND LUNG DISEASE, Jg. 28, Nr. 4, S. 202-203. https://doi.org/10.5588/ijtld.23.0447
Brehm, TT, Reimann, M, Köhler, N & Lange, C 2024, '(Re-)introduction of TNF antagonists and JAK inhibitors in patients with previous tuberculosis: a systematic review', CLINICAL MICROBIOLOGY AND INFECTION , Jg. 30, Nr. 8, S. 989-998. https://doi.org/10.1016/j.cmi.2024.04.011
Brehm, TT, Shijaku, F, Krumkamp, R, Jochum, J, Hoffmann, A, Ramharter, M & Kreuels, B 2024, 'Influenza in travelers from Germany returning from abroad: a retrospective case-control study', BMC INFECTIOUS DISEASES , Jg. 24, Nr. 1, S. 1107. https://doi.org/10.1186/s12879-024-10008-9
Carow, B, Muliadi, V, Skålén, K, Yokota, C, Kathamuthu, GR, Setiabudiawan, TP, Lange, C, Scheu, K, Gaede, KI & Goldmann, T et al. 2024, 'Immune mapping of human tuberculosis and sarcoidosis lung granulomas', FRONTIERS IN IMMUNOLOGY, Jg. 14, S. 1332733. https://doi.org/10.3389/fimmu.2023.1332733
Carratalà-Castro, L, Munguambe, S, Saavedra-Cervera, B, de Haas, P, Kay, A, Marcy, O, Nabeta, P, Ssengooba, W, Ghimenton-Walters, E & Acácio, S et al. 2024, 'Performance of stool-based molecular tests and processing methods for paediatric tuberculosis diagnosis: a systematic review and meta-analysis', The Lancet. Microbe, S. 100963. https://doi.org/10.1016/j.lanmic.2024.100963
Carratalà-Castro, L, Ssengooba, W, Kay, A, Acácio, S, Ehrlich, J, DiNardo, AR, Shiba, N, Nsubuga, JK, Munguambe, S & Saavedra-Cervera, B et al. 2024, 'A stool based qPCR for the diagnosis of TB in children and people living with HIV in Uganda, Eswatini and Mozambique (Stool4TB): a protocol for a multicenter diagnostic evaluation', BMC INFECTIOUS DISEASES , Jg. 24, Nr. 1, S. 233. https://doi.org/10.1186/s12879-023-08708-9
Cirillo, D, Anthony, R, Gagneux, S, Horsburgh, CR, Hasan, R, Darboe, S, Laniado-Laborin, R, Probandari, A, Tukvadze, N & Arcêncio, RA et al. 2024, 'A successful UN High-Level Meeting on antimicrobial resistance must build on the 2023 UN High-Level Meeting on tuberculosis', The Lancet. Global health, Jg. 12, Nr. 8, S. e1225-e1226. https://doi.org/10.1016/S2214-109X(24)00229-8
Dheda, K & Lange, C 2024, 'Towards shorter, safer, flexible, and more effective treatment regimens for drug-resistant tuberculosis', Lancet Respiratory Medicine. https://doi.org/10.1016/S2213-2600(24)00300-X
Dheda, K, Mirzayev, F, Cirillo, DM, Udwadia, Z, Dooley, KE, Chang, K-C, Omar, SV, Reuter, A, Perumal, T & Horsburgh, CR et al. 2024, 'Multidrug-resistant tuberculosis', Nature reviews. Disease primers, Jg. 10, Nr. 1, S. 22. https://doi.org/10.1038/s41572-024-00504-2
Diacon, AH, Barry, CE, Carlton, A, Chen, RY, Davies, M, de Jager, V, Fletcher, K, Koh, GCKW, Kontsevaya, I & Heyckendorf, J et al. 2024, 'A first-in-class leucyl-tRNA synthetase inhibitor, ganfeborole, for rifampicin-susceptible tuberculosis: a phase 2a open-label, randomized trial', NATURE MEDICINE , Jg. 30, Nr. 3, S. 896-904. https://doi.org/10.1038/s41591-024-02829-7
Donald, PR, Kaufmann, SHE, Schaub, D, Thee, S & Lange, C 2024, 'Carl Flügge, one of the last holistic hygienists and discoverer of droplet transmission of infectious diseases', MEDICAL MICROBIOLOGY AND IMMUNOLOGY, Jg. 213, Nr. 1, S. 17. https://doi.org/10.1007/s00430-024-00801-3
Eggeling, J, Kalsdorf, B, Schaub, D, Schierholz, S, Hammerl, P, Nowak, D & Lange, C 2024, 'Alles unter Kontrolle?', Pneumologie (Stuttgart, Germany). https://doi.org/10.1055/a-2313-4137
Eggeling, J, Ramharter, M, Wichmann, D & Schmiedel, S 2024, 'Schwere komplizierte Malaria durch Plasmodium falciparum bei einer Reiserückkehrerin aus Sansibar', Deutsche medizinische Wochenschrift (1946), Jg. 149, Nr. 18, S. 1090-1093. https://doi.org/10.1055/a-2359-7083
Fischer, C, Siakavara, M, Alter, P, Vogelmeier, CF, Speicher, T, Pott, H, Watz, H, Bals, R, Trudzinski, F, Herth, F, Ficker, JH, Wagner, M, Lange, C, Stoycheva, K, Randerath, W, Behr, J, Fähndrich, S, Welte, T, Pink, I, Kahnert, K, Seeger, W, Kuhnert, S, Gessler, T, Adaskina, N & Jörres, RA 2024, 'Association of Patients' Knowledge on the Disease and Its Management with Indicators of Disease Severity and Individual Characteristics in Patients with Chronic Obstructive Pulmonary Disease (COPD): Results from COSYCONET 2', Patient preference and adherence, Jg. 18, S. 2383-2393. https://doi.org/10.2147/PPA.S488165
Gillespie, SH, DiNardo, AR, Georghiou, SB, Sabiiti, W, Kohli, M, Panzner, U, Kontsevaya, I, Hittel, N, Stuyver, LJ & Tan, JB et al. 2024, 'Developing biomarker assays to accelerate tuberculosis drug development: defining target product profiles', The Lancet. Microbe, Jg. 5, Nr. 9, S. 100869. https://doi.org/10.1016/S2666-5247(24)00085-5
Goscé, L, Allel, K, Hamada, Y, Surkova, E, Kontsevaya, I, Wang, TT, Liu, W-H, Matveev, A, Ziganshina, LE & Korobitsyn, A et al. 2024, 'Systematic review of the economic impact of novel Mycobacterium tuberculosis specific antigen-based skin tests for detection of TB infection compared with tuberculin skin test and interferon-gamma release assays', PLOS global public health, Jg. 4, Nr. 10, S. e0003655. https://doi.org/10.1371/journal.pgph.0003655
Günther, G, Guglielmetti, L, Kherabi, Y, Duarte, R, Lange, C & Tuberculosis Network European Trials group 2024, 'Availability of drugs and resistance testing for bedaquiline, pretomanid, linezolid, and moxifloxacin (BPaL(M)) regimen for rifampicin-resistant tuberculosis in Europe', CLINICAL MICROBIOLOGY AND INFECTION , Jg. 30, Nr. 9, S. 1197.e1-1197.e4. https://doi.org/10.1016/j.cmi.2024.03.009
Hauptmann, M, Kalsdorf, B, Akoh-Arrey, JE, Lange, C & Schaible, UE 2024, 'Microbiota alterations in patients treated for susceptible or drug-resistant TB', IJTLD open, Jg. 1, Nr. 8, S. 355-361. https://doi.org/10.5588/ijtldopen.24.0325
Hoelscher, M, Barros-Aguirre, D, Dara, M, Heinrich, N, Sun, E, Lange, C, Tiberi, S & Wells, C 2024, 'Candidate anti-tuberculosis medicines and regimens under clinical evaluation', CLINICAL MICROBIOLOGY AND INFECTION , Jg. 30, Nr. 9, S. 1131-1138. https://doi.org/10.1016/j.cmi.2024.06.016
Holmberg, P, Janoušková, M, Schmidt, T, Neumann, A, Olsson, O, Isberg, P-E, Reimann, M, Riesbeck, K, Skogmar, S & Björkman, P 2024, 'Blood levels of Mycobacterium tuberculosis (Mtb)antigen-triggered immune markers in people exposed to tuberculosis with regard to Mtb infection status and receipt of tuberculosis preventive therapy', Tuberculosis, Jg. 151, S. 102595. https://doi.org/10.1016/j.tube.2024.102595
James, LP, Klaassen, F, Sweeney, S, Furin, J, Franke, MF, Yaesoubi, R, Chesov, D, Ciobanu, N, Codreanu, A & Crudu, V et al. 2024, 'Impact and cost-effectiveness of the 6-month BPaLM regimen for rifampicin-resistant tuberculosis in Moldova: A mathematical modeling analysis', PLoS medicine, Jg. 21, Nr. 5, S. e1004401. https://doi.org/10.1371/journal.pmed.1004401
Kasule, GW, Hermans, S, Semugenze, D, Wekiya, E, Nsubuga, J, Mwachan, P, Kabugo, J, Joloba, M, García-Basteiro, AL & Ssengooba, W et al. 2024, 'Non-sputum-based samples and biomarkers for detection of Mycobacterium tuberculosis: the hope to improve childhood and HIV-associated tuberculosis diagnosis', EUROPEAN JOURNAL OF MEDICAL RESEARCH , Jg. 29, Nr. 1, S. 502. https://doi.org/10.1186/s40001-024-02092-z
Kay, A, Lukhele, B, Dlamini, S, Seeger, A, Dlamini, P, Ndabezitha, S, Mthethwa, N, Steffy, T, Komba, L & Amuge, P et al. 2024, 'Predicting mortality within 1 year of ART initiation in children and adolescents living with HIV in sub-Saharan Africa: a retrospective observational cohort study', The Lancet. Global health, Jg. 12, Nr. 6, S. e929-e937. https://doi.org/10.1016/S2214-109X(24)00091-3
Kay, A, Vasiliu, A, Carratala-Castro, L, Mtafya, B, Mendez Reyes, JE, Maphalala, N, Munguambe, S, Mulengwa, D, Ness, T & Saavedra, B et al. 2024, 'Performance of a stool-based quantitative PCR assay for the diagnosis of tuberculosis in adolescents and adults: a multinational, prospective diagnostic accuracy study', The Lancet. Microbe, Jg. 5, Nr. 5, S. e433-e441. https://doi.org/10.1016/S2666-5247(23)00391-9
Kunst, H, Lange, B, Hovardovska, O, Bockey, A, Zenner, D, Andersen, AB, Hargreaves, S, Pareek, M, Friedland, JS, Wejse, C, Bothamley, G, Guglielmetti, L, Chesov, D, Tiberi, S, Matteelli, A, Mandalakas, AM, Heyckendorf, J, Eimer, J, Malhotra, A, Zamora, J, Vasiliu, A, Lange, C & European Tuberculosis Network TBNET 2024, 'Tuberculosis in adult migrants in Europe: a TBnet consensus statement', The European respiratory journal. https://doi.org/10.1183/13993003.01612-2024
Lange, C, Kherabi, Y, Guglielmetti, L, Duarte, R, Günther, G & Tuberculosis Network European Trials group 2024, 'Availability of drugs and resistance testing for bedaquiline, pretomanid, linezolid, and moxifloxacin (BPaL(M)) regimen for rifampicin-resistant tuberculosis in Europe: author's response', CLINICAL MICROBIOLOGY AND INFECTION , Jg. 30, Nr. 9, S. 1207-1208. https://doi.org/10.1016/j.cmi.2024.06.009
Lange, C, Mandalakas, AM, Lillebaek, T, Chesov, D, Dheda, K & Saluzzo, F 2024, 'Revisiting diagnostics: High priority tuberculosis diagnostic tests that fill an unmet need: what we need and what we don't', CLINICAL MICROBIOLOGY AND INFECTION . https://doi.org/10.1016/j.cmi.2024.12.020
Lienhardt, C, Dooley, KE, Nahid, P, Wells, C, Ryckman, TS, Kendall, EA, Davies, G, Brigden, G, Churchyard, G & Cirillo, DM et al. 2024, 'Target regimen profiles for tuberculosis treatment', Bulletin of the World Health Organization, Jg. 102, Nr. 8, S. 600-607. https://doi.org/10.2471/BLT.24.291881
Loebinger, MR, Aliberti, S, Haworth, C, Jankovic Makek, M, Lange, C, Lorent, N, Papavasileiou, A, Polverino, E, Rohde, G & Veziris, N et al. 2024, 'Patients at risk of nontuberculous mycobacterial pulmonary disease who need testing evaluated using a modified Delphi process by European experts', ERJ Open Research, Jg. 10, Nr. 5. https://doi.org/10.1183/23120541.00791-2023
Martinez, L, Seddon, JA, Horsburgh, CR, Lange, C, Mandalakas, AM & TB Contact Studies Consortium 2024, 'Effectiveness of preventive treatment among different age groups and Mycobacterium tuberculosis infection status: a systematic review and individual-participant data meta-analysis of contact tracing studies', Lancet Respiratory Medicine, Jg. 12, Nr. 8, S. 633-641. https://doi.org/10.1016/S2213-2600(24)00083-3
McClean, M, Panciu, TC, Lange, C, Duarte, R & Theis, F 2024, 'Artificial intelligence in tuberculosis: a new ally in disease control', Breathe, Jg. 20, Nr. 3, S. 240056. https://doi.org/10.1183/20734735.0056-2024
Meiwes, L, Kontsevaya, I, Chesov, D, Kulciţkaia, S, Dreyer, V, Hillemann, D, Dlamini, Q, Williams, C, Barer, M & Brinkmann, F et al. 2024, 'Whispers in the wind: Face mask sampling for Mycobacterium tuberculosis detection in children with pulmonary tuberculosis', JOURNAL OF INFECTIOUS DISEASES. https://doi.org/10.1093/infdis/jiae282
Ness, TE, Cirillo, DM & Mandalakas, AM 2024, 'Commentary: Mixed Infection or Heteroresistance? Pediatric Tuberculosis Still the Achilles Heel of Diagnostic Tools', The Pediatric infectious disease journal. https://doi.org/10.1097/INF.0000000000004648
Neuböck, MJ, and for CPAnet#, Günther, G, Barac, A, Davidsen, JR, Laursen, CB, Agarwal, R, Sehgal, IS, Lange, C & Salzer, HJF 2024, 'Chronic Pulmonary Aspergillosis as a Considerable Complication in Post-Tuberculosis Lung Disease', Seminars in respiratory and critical care medicine, Jg. 45, Nr. 1, S. 102-113. https://doi.org/10.1055/s-0043-1776913
Neumann, M, Reimann, M, Chesov, D, Popa, C, Dragomir, A, Popescu, O, Munteanu, R, Hölscher, A, Honeyborne, I, Heyckendorf, J, Lange, C, Hölscher, C & Kalsdorf, B 2024, 'The Molecular Bacterial Load Assay predicts treatment responses in patients with pre-XDR/XDR-tuberculosis more accurately than GeneXpert Ultra MTB/Rif', Journal of infection, S. 106399. https://doi.org/10.1016/j.jinf.2024.106399
Nieuwenhuizen, NE, Nouailles, G, Sutherland, JS, Zyla, J, Pasternack, AH, Heyckendorf, J, Frye, BC, Höhne, K, Zedler, U & Bandermann, S et al. 2024, 'Activin A levels are raised during human tuberculosis and blockade of the activin signaling axis influences murine responses to M. tuberculosis infection', mBio, Jg. 15, Nr. 3, S. e0340823. https://doi.org/10.1128/mbio.03408-23
Otto-Knapp, R, Bauer, T, Brinkmann, F, Feiterna-Sperling, C, Friesen, I, Geerdes-Fenge, H, Hartmann, P, Häcker, B, Heyckendorf, J & Kuhns, M et al. 2024, 'Treatment of MDR, Pre-XDR, XDR, and Rifampicin-Resistant Tuberculosis or in Case of Intolerance to at Least Rifampicin in Austria, Germany, and Switzerland', RESPIRATION, Jg. 103, Nr. 9, S. 593-600. https://doi.org/10.1159/000539410
Piric, M, Kosan, B, Manke, C, Khreish, F, Fink, L, Koehler, G, Lange, C, Gläser, S, Litzlbauer, D & Markart, P 2024, 'Eine seltene Ursache multipler kavernöser Veränderungen der Lunge', Pneumologie (Stuttgart, Germany). https://doi.org/10.1055/a-2486-6503
Rosenheim, J, Abebe, M, Belay, M, Tulu, B, Tayachew, D, Tegegn, M, Younis, S, Jolliffe, DA, Aseffa, A & Ameni, G et al. 2024, 'Detection of M. tuberculosis DNA in TB contacts' PBMC does not associate with blood RNA signatures for incipient tuberculosis', The European respiratory journal. https://doi.org/10.1183/13993003.00479-2024
Saktiawati, AMI, Vasiliu, A, Saluzzo, F & Akkerman, OW 2024, 'Strategies to Enhance Diagnostic Capabilities for the New Drug-Resistant Tuberculosis (DR-TB) Drugs', Pathogens, Jg. 13, Nr. 12. https://doi.org/10.3390/pathogens13121045
Salzer, HJF & Lange, C 2024, 'Aspergillus-specific IgG antibodies for diagnosing chronic pulmonary aspergillosis compared with the reference standard-author's reply: Aspergillus-specific IgG antibodies for diagnosing chronic pulmonary aspergillosis compared with the reference standard-author's reply', CLINICAL MICROBIOLOGY AND INFECTION , Jg. 30, Nr. 5, S. 696-697. https://doi.org/10.1016/j.cmi.2024.02.010
Schildkraut, JA, Köhler, N, Lange, C, Duarte, R & Gillespie, SH 2024, 'Advances in tuberculosis biomarkers: unravelling risk factors, active disease and treatment success', Breathe, Jg. 20, Nr. 3, S. 240003. https://doi.org/10.1183/20734735.0003-2024
Schönfeld, N, Barkane, L, Davoliene, I, Danilovits, M, Miliauskas, S, Ader, F, Kon, OM, Lange, C, Duvignaud, A & Heiss-Neumann, M et al. 2024, 'Real-life use of delamanid: results from the European post-authorisation safety study', IJTLD open, Jg. 1, Nr. 6, S. 274-278. https://doi.org/10.5588/ijtldopen.24.0113
Shaw, ES, Stoker, NG, Potter, JL, Claassen, H, Leslie, A, Tweed, CD, Chiang, C-Y, Conradie, F, Esmail, H & Lange, C et al. 2024, 'Bedaquiline: what might the future hold?', The Lancet. Microbe, S. 100909. https://doi.org/10.1016/S2666-5247(24)00149-6
Stoycheva, K, Cristea, V, Ködmön, C, Rosales-Klintz, S, Zenner, D, Vasiliu, A, van der Werf, M & Lange, C 2024, 'Tuberculosis in people of Ukrainian origin in the European Union and the European Economic Area, 2019 to 2022', Eurosurveillance, Jg. 29, Nr. 12, 2400094. https://doi.org/10.2807/1560-7917.ES.2024.29.12.2400094
Sutter, JP, Kocheise, L, Kempski, J, Christner, M, Wichmann, D, Pinnschmidt, H, Schmiedel, S, Lohse, AW, Huber, S & Brehm, TT 2024, 'Gentamicin combination treatment is associated with lower mortality in patients with invasive listeriosis: a retrospective analysis', Infection, Jg. 52, Nr. 4, S. 1601-1606. https://doi.org/10.1007/s15010-024-02330-w
Sutter, JP, Kocheise, L, Kempski, J, Christner, M, Wichmann, D, Pinnschmidt, H, Schmiedel, S, Lohse, AW, Huber, S & Brehm, TT 2024, 'Reply to: Challenges in interpreting the role of gentamicin in treatment of invasive listeriosis: immortal timebias and confounding', Infection. https://doi.org/10.1007/s15010-024-02417-4
Wetzstein, N, Dahl, VN, Lillebaek, T & Lange, C 2024, 'Clinical spectrum and relevance of Mycobacterium malmoense: Systematic review and meta-analysis of 859 patients', Journal of infection, Jg. 89, Nr. 2, S. 106203. https://doi.org/10.1016/j.jinf.2024.106203
Zielinski, N, Baiceanu, D, Dragomir, A, Heyckendorf, J, Ibraim, E, Köhler, N, Leschczyk, C, Popa, C, Rachow, A & Sachsenweger, J et al. 2024, 'A Transcriptomic Biomarker Predicting Linezolid-Associated Neuropathy During Treatment of Drug-Resistant Tuberculosis', Pathogens and Immunity, Jg. 9, Nr. 2, S. 25-42. https://doi.org/10.20411/pai.v9i2.705
2023
Brehm, TT, Köhler, N, Schmiedel, S, Terhalle, E, Martensen, J, Kalsdorf, B, Kandulla, J, Heyckendorf, J, Kuhns, M, Friesen, I & Lange, C 2023, 'Therapie der Tuberkulose: Was gibt es Neues?', Innere Medizin (Heidelberg, Germany), Jg. 64, Nr. 7, S. 701-707. https://doi.org/10.1007/s00108-023-01523-z
Butov, D, Feshchenko, Y, Chesov, D, Myasoedov, V, Kuzhko, M, Dudnyk, A, Reimann, M, Hryshchuk, L, Yareshko, A, Tkachenko, A, Tarleeva, Y, Konstantynovska, O, Butova, T & Lange, C 2023, 'National survey on the impact of the war in Ukraine on TB diagnostics and treatment services in 2022', INTERNATIONAL JOURNAL OF TUBERCULOSIS AND LUNG DISEASE, Jg. 27, Nr. 1, S. 86-88. https://doi.org/10.5588/ijtld.22.0563
Chesov, E, Chesov, D, Reimann, M, Dreyer, V, Utpatel, C, Gröschel, MI, Ciobanu, N, Crudu, V, Lange, C, Heyckendorf, J & Merker, M 2023, 'Impact of Mycobacterium tuberculosis strain type on multidrug-resistant tuberculosis severity, Republic of Moldova', Journal of infection, Jg. 87, Nr. 6, S. 588-591. https://doi.org/10.1016/j.jinf.2023.10.001
European Tuberculosis Network TBNET, Günther, G, Guglielmetti, L, Leu, C, Lange, C & van Leth, F 2023, 'Availability and costs of medicines for the treatment of tuberculosis in Europe', CLINICAL MICROBIOLOGY AND INFECTION , Jg. 29, Nr. 1, S. 77-84. https://doi.org/10.1016/j.cmi.2022.07.026
Günther, G, Guglielmetti, L, Leu, C, van Leth, F & Lange, C 2023, 'Relative cost of multidrug-resistant TB medicines in Europe', INTERNATIONAL JOURNAL OF TUBERCULOSIS AND LUNG DISEASE, Jg. 27, Nr. 5, S. 341-344. https://doi.org/10.5588/ijtld.23.0026
Hamada, Y, Gupta, RK, Quartagno, M, Izzard, A, Acuna-Villaorduna, C, Altet, N, Diel, R, Dominguez, J, Floyd, S, Gupta, A, Huerga, H, Jones-López, EC, Kinikar, A, Lange, C, van Leth, F, Liu, Q, Lu, W, Lu, P, Rueda, IL, Martinez, L, Mbandi, SK, Muñoz, L, Padilla, ES, Paradkar, M, Scriba, T, Sester, M, Shanaube, K, Sharma, SK, Sloot, R, Sotgiu, G, Thiruvengadam, K, Vashishtha, R, Abubakar, I & Rangaka, MX 2023, 'Predictive performance of interferon-gamma release assays and the tuberculin skin test for incident tuberculosis: an individual participant data meta-analysis', EClinicalMedicine, Jg. 56, S. 101815. https://doi.org/10.1016/j.eclinm.2022.101815
Hamada, Y, Kontsevaya, I, Surkova, E, Wang, TT, Wan-Hsin, L, Matveev, A, Ziganshina, LE, Denkinger, CM, Korobitsyn, A, Ismail, N, Abubakar, I & Rangaka, MX 2023, 'A Systematic Review on the Safety of Mycobacterium tuberculosis-Specific Antigen-Based Skin Tests for Tuberculosis Infection Compared With Tuberculin Skin Tests', Open forum infectious diseases, Jg. 10, Nr. 5, S. ofad228. https://doi.org/10.1093/ofid/ofad228
Ivanova, O, Hoffmann, VS, Lange, C, Hoelscher, M & Rachow, A 2023, 'Post-tuberculosis lung impairment: systematic review and meta-analysis of spirometry data from 14 621 people', European Respiratory Review, Jg. 32, Nr. 168, S. 220221. https://doi.org/10.1183/16000617.0221-2022
Koehler, N, Andres, S, Merker, M, Dreyer, V, John, A, Kuhns, M, Krieger, D, Choong, E, Verougstraete, N, Zur Wiesch, PA, Wicha, SG, König, C, Kalsdorf, B, Sanchez Carballo, PM, Schaub, D, Werngren, J, Schön, T, Peloquin, CA, Schönfeld, N, Verstraete, AG, Decosterd, LA, Aarnoutse, R, Niemann, S, Maurer, FP & Lange, C 2023, 'Pretomanid-Resistant Tuberculosis', Journal of infection, Jg. 86, Nr. 5, S. 520-524. https://doi.org/10.1016/j.jinf.2023.01.039
Kontsevaya, I, Cabibbe, AM, Cirillo, DM, DiNardo, AR, Frahm, N, Gillespie, SH, Holtzman, D, Meiwes, L, Petruccioli, E, Reimann, M, Ruhwald, M, Sabiiti, W, Saluzzo, F, Tagliani, E & Goletti, D 2023, 'Update on the diagnosis of tuberculosis', CLINICAL MICROBIOLOGY AND INFECTION . https://doi.org/10.1016/j.cmi.2023.07.014
Lange, C 2023, 'Management der antibiotikaresistenten Tuberkulose', Deutsche medizinische Wochenschrift (1946), Jg. 148, Nr. 19, S. 1236-1241. https://doi.org/10.1055/a-1939-0000
Lange, C 2023, 'Mission (im)possible: elimination of tuberculosis', CLINICAL MICROBIOLOGY AND INFECTION . https://doi.org/10.1016/j.cmi.2023.07.033
Lange, C, Brehm, TT, Chesov, D, Kherabi, Y & Guglielmetti, L 2023, Treatment of drug-susceptible and drug-resistant tuberculosis. European Respiratory Society. https://doi.org/10.1183/2312508X.10024622
Lange, C, Köhler, N & Günther, G 2023, 'Regimens for Drug-Resistant Tuberculosis', New England Journal of Medicine, Jg. 388, Nr. 2, S. 189-191. https://doi.org/10.1056/NEJMc2213970
Lange, C, Vasiliu, A & Mandalakas, AM 2023, 'Emerging bedaquiline-resistant tuberculosis', The Lancet. Microbe, Jg. 4, Nr. 12, S. e964-e965. https://doi.org/10.1016/S2666-5247(23)00321-X
Maier, C, Chesov, D, Schaub, D, Kalsdorf, B, Andres, S, Friesen, I, Reimann, M & Lange, C 2023, 'Long-term treatment outcomes in patients with multidrug-resistant tuberculosis', CLINICAL MICROBIOLOGY AND INFECTION , Jg. 29, Nr. 6, S. 751-757. https://doi.org/10.1016/j.cmi.2023.02.013
Motta, I, Boeree, M, Chesov, D, Dheda, K, Günther, G, Horsburgh, CR, Kherabi, Y, Lange, C, Lienhardt, C, McIlleron, HM, Paton, NI, Stagg, HR, Thwaites, G, Udwadia, Z, Van Crevel, R, Velásquez, GE, Wilkinson, RJ & Guglielmetti, L 2023, 'Recent advances in the treatment of tuberculosis', CLINICAL MICROBIOLOGY AND INFECTION . https://doi.org/10.1016/j.cmi.2023.07.013
Ness, T, Van, LH, Petermane, I, Duarte, R, Lange, C, Menzies, D & Cirillo, DM 2023, 'Rolling out new anti-tuberculosis drugs without diagnostic capacity', Breathe, Jg. 19, Nr. 2, S. 230084. https://doi.org/10.1183/20734735.0084-2023
Ness, TE, Meiwes, L, Kay, A, Mejia, R, Lange, C, Farhat, M, Mandalakas, A & DiNardo, A 2023, 'Optimizing DNA Extraction from Pediatric Stool for Diagnosis of Tuberculosis and Use in Next-Generation Sequencing Applications', Microbiology spectrum, Jg. 11, Nr. 1, S. e0226922. https://doi.org/10.1128/spectrum.02269-22
Noroc, E, Chesov, D, Merker, M, Gröschel, MI, Barilar, I, Dreyer, V, Ciobanu, N, Reimann, M, Crudu, V & Lange, C 2023, 'Limited Nosocomial Transmission of Drug-Resistant Tuberculosis, Moldova', EMERGING INFECTIOUS DISEASES, Jg. 29, Nr. 5, S. 1046-1050. https://doi.org/10.3201/eid2905.230035
Pedersen, OS, Holmgaard, FB, Mikkelsen, MKD, Lange, C, Sotgiu, G, Lillebaek, T, Andersen, AB, Wejse, CM & Dahl, VN 2023, 'Global treatment outcomes of extensively drug-resistant tuberculosis in adults: A systematic review and meta-analysis', Journal of infection, Jg. 87, Nr. 3, S. 177-189. https://doi.org/10.1016/j.jinf.2023.06.014
Sandoval, M, Mtetwa, G, Devezin, T, Vambe, D, Sibanda, J, Dube, GS, Dlamini-Simelane, T, Lukhele, B, Mandalakas, AM & Kay, A 2023, 'Community-based tuberculosis contact management: Caregiver experience and factors promoting adherence to preventive therapy', PLOS global public health, Jg. 3, Nr. 7, S. e0001920. https://doi.org/10.1371/journal.pgph.0001920
TBNET and RESIST-TB networks, Domínguez, J, Boeree, MJ, Cambau, E, Chesov, D, Conradie, F, Cox, V, Dheda, K, Dudnyk, A, Farhat, MR, Gagneux, S, Grobusch, MP, Gröschel, MI, Guglielmetti, L, Kontsevaya, I, Lange, B, van Leth, F, Lienhardt, C, Mandalakas, AM, Maurer, FP, Merker, M, Miotto, P, Molina-Moya, B, Morel, F, Niemann, S, Veziris, N, Whitelaw, A, Horsburgh, CR & Lange, C 2023, 'Clinical implications of molecular drug resistance testing for Mycobacterium tuberculosis: a 2023 TBnet/RESIST-TB consensus statement', Lancet Infectious Diseases, Jg. 23, Nr. 4, S. e122-e137. https://doi.org/10.1016/S1473-3099(22)00875-1
UNITE4TB Consortium 2023, 'Implementing molecular tuberculosis diagnostic methods in limited-resource and high-burden countries', Breathe, Jg. 18, Nr. 4, S. 220226. https://doi.org/10.1183/20734735.0226-2022
UNITE4TB Consortium 2023, 'Tuberculosis: current challenges and beyond', Breathe, Jg. 19, Nr. 1, S. 220166. https://doi.org/10.1183/20734735.0166-2022
2022
Akkerman, OW, Duarte, R, Tiberi, S, Schaaf, HS, Lange, C, Alffenaar, JWC, Denholm, J, Carvalho, ACC, Bolhuis, MS, Borisov, S, Bruchfeld, J, Cabibbe, AM, Caminero, JA, Carvalho, I, Chakaya, J, Centis, R, Dalcomo, MP, D Ambrosio, L, Dedicoat, M, Dheda, K, Dooley, KE, Furin, J, García-García, J-M, van Hest, NAH, de Jong, BC, Kurhasani, X, Märtson, AG, Mpagama, S, Torrico, MM, Nunes, E, Ong, CWM, Palmero, DJ, Ruslami, R, Saktiawati, AMI, Semuto, C, Silva, DR, Singla, R, Solovic, I, Srivastava, S, de Steenwinkel, JEM, Story, A, Sturkenboom, MGG, Tadolini, M, Udwadia, ZF, Verhage, AR, Zellweger, JP & Migliori, GB 2022, 'Clinical standards for drug-susceptible pulmonary TB', INTERNATIONAL JOURNAL OF TUBERCULOSIS AND LUNG DISEASE, Jg. 26, Nr. 7, S. 592-604. https://doi.org/10.5588/ijtld.22.0228
Brandenburg, J, Heyckendorf, J, Marwitz, F, Zehethofer, N, Linnemann, L, Gisch, N, Karaköse, H, Reimann, M, Kranzer, K, Kalsdorf, B, Sanchez-Carballo, P, Weinkauf, M, Scholz, V, Malm, S, Homolka, S, Gaede, KI, Herzmann, C, Schaible, UE, Hölscher, C, Reiling, N & Schwudke, D 2022, 'Tuberculostearic Acid-Containing Phosphatidylinositols as Markers of Bacterial Burden in Tuberculosis', ACS infectious diseases, Jg. 8, Nr. 7, S. 1303-1315. https://doi.org/10.1021/acsinfecdis.2c00075
Chesov, E, Chesov, D, Maurer, FP, Andres, S, Utpatel, C, Barilar, I, Donica, A, Reimann, M, Niemann, S, Lange, C, Crudu, V, Heyckendorf, J & Merker, M 2022, 'Emergence of bedaquiline resistance in a high tuberculosis burden country', The European respiratory journal, Jg. 59, Nr. 3, 2100621. https://doi.org/10.1183/13993003.00621-2021
COVICAT study group, Aachen Study (COVAS) 2022, 'Detailed stratified GWAS analysis for severe COVID-19 in four European populations', HUMAN MOLECULAR GENETICS , Jg. 31, Nr. 23, S. 3945-3966. https://doi.org/10.1093/hmg/ddac158
Donald, P, Kaufmann, S, Thee, S, Mandalakas, AM & Lange, C 2022, 'Pathogenesis of tuberculosis: the 1930 Lübeck disaster revisited', European Respiratory Review, Jg. 31, Nr. 164, 220046. https://doi.org/10.1183/16000617.0046-2022
DZIF-TB cohort study group 2022, 'Gene expression signatures identify biologically and clinically distinct tuberculosis endotypes', The European respiratory journal, Jg. 60, Nr. 3. https://doi.org/10.1183/13993003.02263-2021
European Conference on Infections in Leukaemia group 2022, 'Mycobacterial infections in adults with haematological malignancies and haematopoietic stem cell transplants: guidelines from the 8th European Conference on Infections in Leukaemia', Lancet Infectious Diseases, Jg. 22, Nr. 12, S. e359-e369. https://doi.org/10.1016/S1473-3099(22)00227-4
expert panel group for management recommendations in non-tuberculous mycobacterial pulmonary diseases 2022, 'Consensus management recommendations for less common non-tuberculous mycobacterial pulmonary diseases', Lancet Infectious Diseases, Jg. 22, Nr. 7, S. e178-e190. https://doi.org/10.1016/S1473-3099(21)00586-7
Günther, G, Saathoff, E, Rachow, A, Ekandjo, H, Diergaardt, A, Marais, N, Lange, C & Nepolo, E 2022, 'Clinical Evaluation of a Line-Probe Assay for Tuberculosis Detection and Drug-Resistance Prediction in Namibia', Microbiology spectrum, Jg. 10, Nr. 3, S. e0025922. https://doi.org/10.1128/spectrum.00259-22
Gutowski, J-P, Sachsenweger, J, May, K, Lange, C & Heyckendorf, J 2022, 'Eine unerwartete Komplikation: Kontralateraler Spannungspneumothorax nach transbronchialer Zangenbiopsie', Pneumologie (Stuttgart, Germany), Jg. 76, Nr. 9, S. 622-625. https://doi.org/10.1055/a-1775-5693
Lange, C, Aaby, P, Behr, MA, Donald, PR, Kaufmann, SHE, Netea, MG & Mandalakas, AM 2022, '100 years of Mycobacterium bovis bacille Calmette-Guérin', Lancet Infectious Diseases, Jg. 22, Nr. 1, S. e2-e12. https://doi.org/10.1016/S1473-3099(21)00403-5
Lange, C, Kay, A & Mandalakas, AM 2022, 'The need for effective drugs for TB prevention: set your goals high, and don´t stop till you get there', INTERNATIONAL JOURNAL OF TUBERCULOSIS AND LUNG DISEASE, Jg. 26, Nr. 2, S. 85-88. https://doi.org/10.5588/ijtld.21.0736
Martinez, L, Cords, O, Liu, Q, Acuna-Villaorduna, C, Bonnet, M, Fox, GJ, Carvalho, ACC, Chan, P-C, Croda, J, Hill, PC, Lopez-Varela, E, Donkor, S, Fielding, K, Graham, SM, Espinal, MA, Kampmann, B, Reingold, A, Huerga, H, Villalba, JA, Grandjean, L, Sotgiu, G, Egere, U, Singh, S, Zhu, L, Lienhardt, C, Denholm, JT, Seddon, JA, Whalen, CC, García-Basteiro, AL, Triasih, R, Chen, C, Singh, J, Huang, L-M, Sharma, S, Hannoun, D, Del Corral, H, Mandalakas, AM, Malone, LL, Ling, D-L, Kritski, A, Stein, CM, Vashishtha, R, Boulahbal, F, Fang, C-T, Boom, WH, Netto, EM, Lemos, AC, Hesseling, AC, Kay, A, Jones-López, EC, Horsburgh, CR, Lange, C & Andrews, JR 2022, 'Infant BCG vaccination and risk of pulmonary and extrapulmonary tuberculosis throughout the life course: a systematic review and individual participant data meta-analysis', The Lancet. Global health, Jg. 10, Nr. 9, S. e1307-e1316. https://doi.org/10.1016/S2214-109X(22)00283-2
Mercier, T, Desfontaine, V, Cruchon, S, Da Silva Pereira Clara, JA, Briki, M, Mazza-Stalder, J, Kajkus, A, Burger, R, Suttels, V, Buclin, T, Opota, O, Koehler, N, Sanchez Carballo, PM, Lange, C, André, P, Decosterd, LA & Choong, E 2022, 'A battery of tandem mass spectrometry assays with stable isotope-dilution for the quantification of 15 anti-tuberculosis drugs and two metabolites in patients with susceptible-, multidrug-resistant- and extensively drug-resistant tuberculosis', JOURNAL OF CHROMATOGRAPHY B-ANALYTICAL TECHNOLOGIES IN THE BIOMEDICAL AND LIFE SCIENCES, Jg. 1211, S. 123456. https://doi.org/10.1016/j.jchromb.2022.123456
Rachow, A, Saathoff, E, Mindru, R, Popescu, O, Lugoji, D, Mahler, B, Merker, M, Niemann, S, Olaru, ID, Kastner, S, Hoelscher, M, Lange, C & Ibraim, E 2022, 'Diagnostic performance of the AID line probe assay in the detection of Mycobacterium tuberculosis and drug resistance in Romanian patients with presumed TB', PLOS ONE, Jg. 17, Nr. 8, S. e0271297. https://doi.org/10.1371/journal.pone.0271297
Ramachandran, R, Dumitrescu, A, Baiceanu, D, Popa, C, Dragomir, A, Mahler, B, Hoelscher, M, Lange, C, Heyckendorf, J, Rachow, A, Ibraim, E & Ivanova, O 2024, 'Impact of drug-resistant tuberculosis on socio-economic status, quality of life and psychological well-being of patients in Bucharest, Romania: a prospective cohort study', Journal of health, population, and nutrition, Jg. 43, Nr. 1, S. 223. https://doi.org/10.1186/s41043-024-00717-x
Rauch, A, Köhler, N, Brehm, TT, Zielinski, N, Stoycheva, K, Maier, C, Böttcher, L, Friesen, I, Schaub, D, Reimann, M, Schmiedel, S, Lange, C & Kalsdorf, B 2024, 'Long-Term Self-Administered Outpatient Parenteral Antimicrobial Therapy in the Treatment of Tuberculosis', Drugs. https://doi.org/10.1007/s40265-024-02122-4
Schaberg, T, Brinkmann, F, Feiterna-Sperling, C, Geerdes-Fenge, H, Hartmann, P, Häcker, B, Hauer, B, Haas, W, Heyckendorf, J, Lange, C, Maurer, FP, Nienhaus, A, Otto-Knapp, R, Priwitzer, M, Richter, E, Salzer, HJF, Schoch, O, Schönfeld, N, Stahlmann, R & Bauer, T 2022, 'Tuberkulose im Erwachsenenalter', Pneumologie (Stuttgart, Germany), Jg. 76, Nr. 11, S. 727-819. https://doi.org/10.1055/a-1934-8303
Sibandze, DB, Kay, A, Dreyer, V, Sikhondze, W, Dlamini, Q, DiNardo, A, Mtetwa, G, Lukhele, B, Vambe, D, Lange, C, Glenn Dlamini, M, Ness, T, Mejia, R, Kalsdorf, B, Heyckendorf, J, Kuhns, M, Maurer, FP, Dlamini, S, Maphalala, G, Niemann, S & Mandalakas, A 2022, 'Rapid molecular diagnostics of tuberculosis resistance by targeted stool sequencing', Genome medicine, Jg. 14, Nr. 1, S. 52. https://doi.org/10.1186/s13073-022-01054-6
Study Group on Mycobacteria of the European Society of Microbiology and Infectious Diseases (ESGMYC), European Society of Mycobacteriology (ESM), European Respiratory Society (ERS) and 2022, 'Rifapentine access in Europe: growing concerns over key tuberculosis treatment component', The European respiratory journal, Jg. 59, Nr. 5, S. 2200388. https://doi.org/10.1183/13993003.00388-2022
TBnet, The ESGMYC, and The French MDR-TB Group 2022, 'Co-administration of treatment for rifampicin-resistant TB and chronic HCV infection: a TBnet and ESGMYC study', Journal of infection, Jg. 84, Nr. 6, S. 834-872. https://doi.org/10.1016/j.jinf.2022.03.004
Tran, F, Harris, DMM, Scharmacher, A, Graßhoff, H, Sterner, K, Schinke, S, Käding, N, Humrich, JY, Cabral-Marques, O, Bernardes, JP, Mishra, N, Bahmer, T, Franzenburg, J, Hoyer, BF, Glück, A, Guggeis, M, Ossysek, A, Küller, A, Frank, D, Lange, C, Rupp, J, Heyckendorf, J, Gaede, KI, Amital, H, Rosenstiel, P, Shoenfeld, Y, Halpert, G, Rosenberg, AZ, Schulze-Forster, K, Heidecke, H, Riemekasten, G & Schreiber, S 2022, 'Increased protease-activated receptor 1 autoantibodies are associated with severe COVID-19', ERJ Open Research, Jg. 8, Nr. 4. https://doi.org/10.1183/23120541.00379-2022
UNITE4TB Consortium 2022, 'Tuberculosis Treatment Monitoring and Outcome Measures: New Interest and New Strategies', Clinical microbiology reviews, Jg. 35, Nr. 3, S. e0022721. https://doi.org/10.1128/cmr.00227-21
Head

Scientific staff



















Project Management

Technical staff




Data Monitoring